8 Climate Regulation
We think of the atmosphere as essential for life because it provides oxygen for us to breathe, but without it, Earth would not be habitable at all: without an atmosphere, our planet would be about 30°C colder than it is, and completely frozen over. In this chapter we will explore the combined roles of the atmosphere and plate tectonics in regulating Earth’s climate.
Learning Objectives
By the end of this chapter, you will be able to:
- describe the contents of Earth’s atmosphere
- explain how the greenhouse effect warms the Earth
- describe the inorganic carbon cycle and its relationship to plate tectonics
- explain how the greenhouse effect and the carbon cycle work together to stabilize Earth’s climate
Composition of the Atmosphere
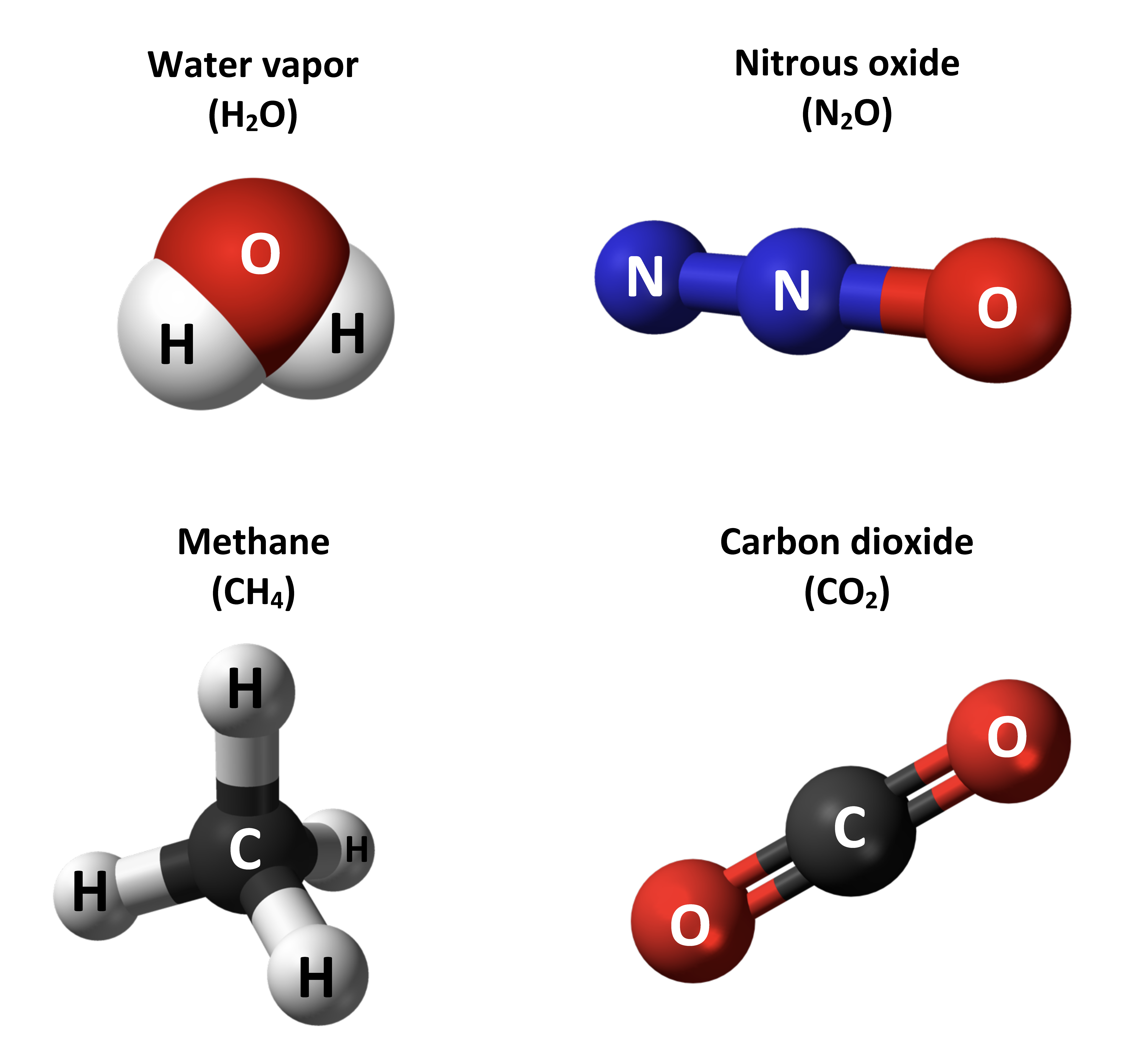
Earth’s temperature depends on the composition of the atmosphere. The primary components are nitrogen (N2, 78%) and oxygen (O2, 21%), and the remainder is argon (Ar, about 1%) and trace amounts of other gases. Among those other gases are the greenhouse gases that make Earth habitable. The most common greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), as shown in Figure 1. Note that they all contain at least three atoms.
Water vapor is the most abundant greenhouse gas, but its atmospheric abundance does not change much over time. Carbon dioxide is much less abundant than water vapor, but it is currently being added to the atmosphere by human activities such as burning fossil fuels, land-use changes, and deforestation. Carbon dioxide stays in the atmosphere for hundreds of years. Although there is much less methane than carbon dioxide in the atmosphere, it is 30 times more effective as a greenhouse gas. It is also removed from the atmosphere much more quickly.
What makes a gas a greenhouse gas?
The dominant gases of the atmosphere are nitrogen (as N2) and oxygen (as O2). These gas molecules have only two atoms each, and are not greenhouse gases. In contrast, water vapor (H2O), carbon dioxide (CO2), and methane (CH4) all have more than two atoms, and they are greenhouse gases. Why does the number of atoms matter?
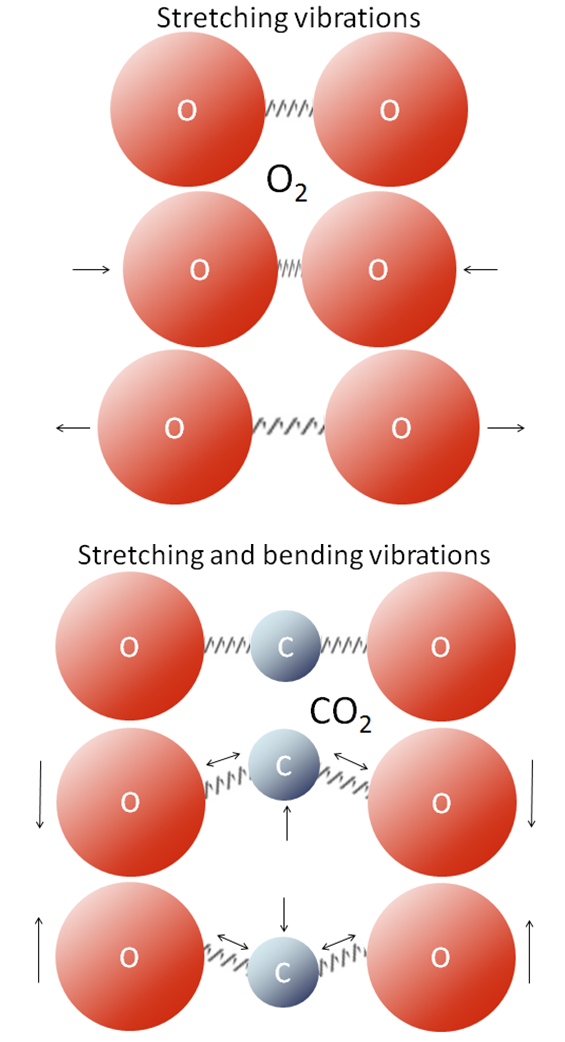
All molecules vibrate at various frequencies and in various ways, and some of those vibrations take place at frequencies within the range of the infrared (IR) radiation that is emitted by Earth’s surface. Gases with two atoms, such as O2, can only vibrate by stretching (back and forth; Figure 2 top), and those vibrations are much faster than the frequencies of IR radiation. Gases with three or more atoms (such as CO2) vibrate by stretching as well, but they can also vibrate in other ways, such as by bending (Figure 2 bottom). Those vibrations are slower and allow the molecules to absorb and release infrared radiation.
When infrared radiation interacts with CO2 or with one of the other greenhouse gases, the molecular vibrations are enhanced because there is a match between the wavelength of the IR light and the vibrational frequency of the molecule. This makes the molecule vibrate more vigorously, heating the surrounding air in the process. These molecules also emit IR radiation in all directions, some of which reaches Earth’s surface. The heating caused by the more vigorous vibrations of greenhouse gases and their emission of IR radiation is called the greenhouse effect. Water molecules (H2O), and methane molecules (CH4) also interact with infrared radiation when they vibrate, so they are greenhouse gases as well.
Greenhouse gases trap heat in the atmosphere and warm the planet by absorbing some of the infrared radiation that is emitted by the Earth, thus keeping that heat from being lost to space. More greenhouse gases in the atmosphere absorb more heat and make the planet warmer.
The Greenhouse Effect
The greenhouse effect is named after the process that warms a greenhouse or a car on a hot summer day: sunlight passes through the glass of the greenhouse or car, reaches the interior, and is absorbed and reradiated as heat. The heat radiates upward, but gets trapped by the glass windows instead of escaping. On Earth, greenhouse gases in the atmosphere play the role of the windows. The greenhouse effect can be explained in three steps.
Step 1: Radiation from the Sun strikes the Earth. This radiation is composed of mostly ultraviolet (UV), visible, and infrared (IR) light. Part of the radiation is directly reflected by Earth’s surface or atmosphere, and part is immediately absorbed by greenhouse gases in the atmosphere. About half of the solar radiation is absorbed by the Earth’s surface.
Step 2: The visible, UV, and IR radiation that reaches the surface is converted to into heat. You have probably experienced sunlight warming a surface such as pavement, a patio, or deck. When this happens, the warmed surface then emits thermal IR radiation: heat. In this way, the visible, UV, and IR light absorbed by Earth’s surface is converted to only IR, and reradiated by the surface.
Step 3: The energy absorbed by the Earth’s surface radiates back into the atmosphere, but now only at IR wavelengths. If there were no atmosphere, this IR radiation would simply escape into space. Instead, it encounters greenhouse gas molecules in the atmosphere, is absorbed by those molecules, and is finally reemitted once again. The IR radiation emitted by the greenhouse gas molecules provides the additional source of heat that warms the Earth. The gases that are mostly responsible for this process include carbon dioxide, water vapor, methane, and nitrous oxide. The presence of more greenhouse gases in the atmosphere results in more thermal IR being trapped, and more heating. The animation below shows a simplified version of the greenhouse effect.

Earth’s Energy Budget
The solar radiation that reaches Earth is relatively uniform over time. Earth is warmed, and energy or heat radiates from the Earth’s surface and lower atmosphere back to space. This flow of incoming and outgoing energy is Earth’s energy budget. For Earth’s temperature to be stable over long stretches of time, incoming energy and outgoing energy have to be equal on average, balancing the energy budget at the top of the atmosphere. About 29% of the incoming solar energy arriving at the top of the atmosphere is reflected back to space by clouds, atmospheric particles, or reflective ground surfaces like sea ice and snow. About 23% of incoming solar energy is absorbed in the atmosphere by water vapor, dust, and ozone. The remaining 48% passes through the atmosphere and is absorbed at the surface. Thus, about 71% of the total incoming solar energy is absorbed by the Earth system (Figure 4).
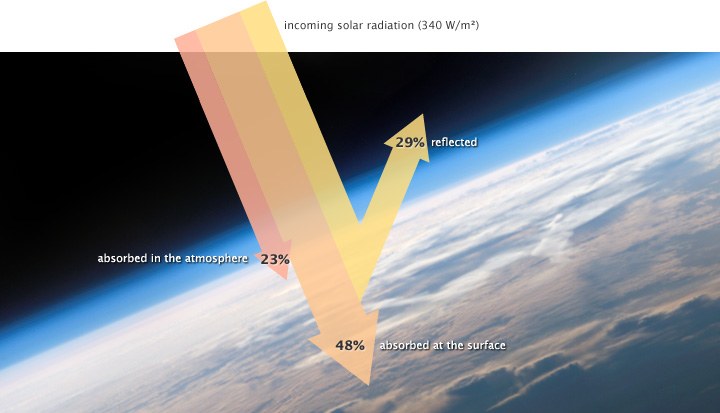
When this energy reaches Earth, the atoms and molecules that make up the atmosphere and surface absorb the energy, and Earth’s temperature increases. If this material only absorbed energy, then the temperature of the Earth would continue to increase and eventually overheat. For example, if you continuously run a faucet in a stopped-up sink, the water level rises and eventually overflows. However, temperature does not infinitely rise because the Earth is not just absorbing sunlight; it is also radiating thermal energy or heat back into the atmosphere. If the temperature of the Earth rises, the planet emits an increasing amount of heat to space. This is the primary mechanism that prevents Earth from continually heating.
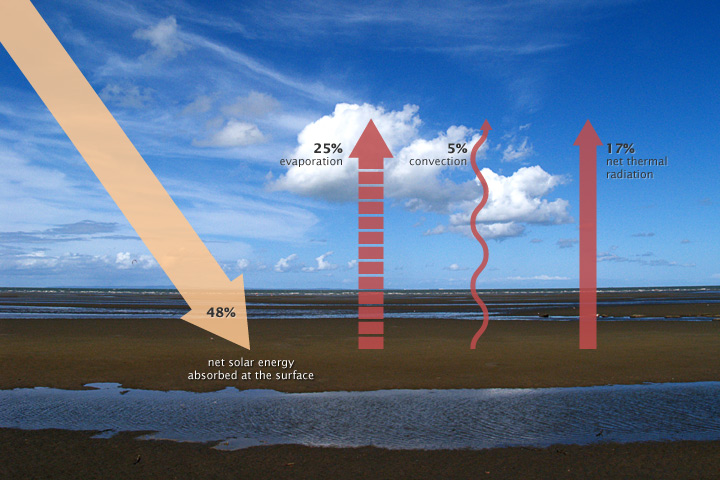
Some of the thermal infrared heat radiating from the surface is absorbed and trapped by greenhouse gases in the atmosphere, which act like a giant canopy over Earth. The more greenhouse gases in the atmosphere, the more outgoing heat Earth retains and the less thermal infrared heat dissipates to space.
Factors beyond greenhouse gases can affect the Earth’s energy budget. Increasing or decreasing solar energy will change the energy Earth receives. Such changes can be due to long term evolution of the Sun, as it grows brighter with age, or to changes in the orbit of the Earth that alter the distance to the Sun. Orbital variations are largely responsible for Earth’s recent ice ages. These changes due to the Sun are very small over time. In addition, land and water will absorb more sunlight when there is less ice and snow to reflect the sunlight back to the atmosphere. For example, the ice covering the Arctic Sea reflects sunlight back to the atmosphere. Furthermore, aerosols (dust particles) produced from burning coal, diesel engines, and volcanic eruptions can reflect incoming solar radiation and actually cool the planet.
The Carbon Cycle
Most of Earth’s carbon dioxide is not in the atmosphere. Instead, it is locked in carbonate rocks, sedimentary rocks such as limestone that are rich in carbon. Plate tectonics plays a key role in both locking carbon in rocks and in regulating the carbon dioxide content of the atmosphere. The inorganic or geological carbon cycle stabilizes our climate thanks to plate tectonics and chemical interactions between surface rocks and the atmosphere (this is also called the slow carbon cycle because it takes place over geological time—the fast or biological carbon cycle involves the flow of carbon between the atmosphere and Earth’s ecosystems).
As shown in Figure 6, carbon dioxide in the atmosphere dissolves in rainwater, creating carbonic acid. This weak acid reacts with rocks, slowly breaking them down and carrying the dissolved minerals to the oceans. Over time, carbon dioxide in the oceans reacts with the dissolved minerals to create new carbonate rocks on the ocean floor. These rocks are then transported into the deep mantle in subduction zones, where they eventually melt, releasing CO2. The CO2 is released back into the atmosphere by volcanoes, completing the cycle. In summary, plate tectonics on Earth allows carbon to be cycled from the atmosphere, down to carbonate rocks on the ocean floor, and back into the atmosphere through volcanism.
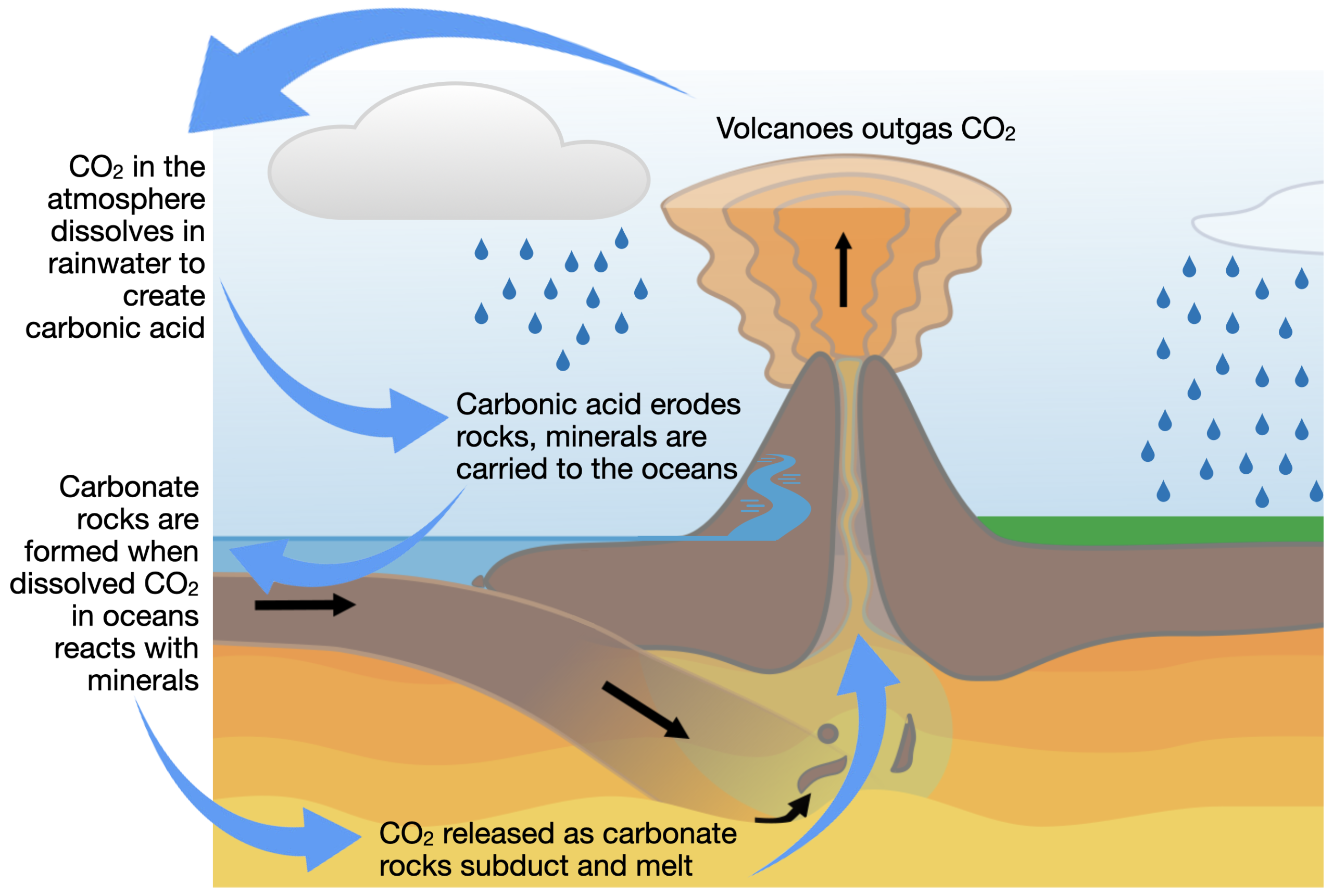
This carbon cycle is an essential part of maintaining a hospitable atmosphere on Earth, and it would not be possible without liquid water and plate tectonics. Other planets in our solar system experience tectonic stresses, but plate tectonics – with the active movement of crustal and oceanic plates – is unique to Earth. When exploring exoplanets that may be conducive to life, the presence of plate tectonics would be a favorable condition, at least based on our experience on Earth.
Climate Regulation
The greenhouse effect and the carbon cycle work together in a negative, stabilizing feedback loop that maintains Earth’s temperature. To see how this works, we can imagine raising Earth’s temperature. This change will increase evaporation from the oceans, thus increasing the amount of CO2 that is removed from the atmosphere by dissolution in rainwater. An increase in temperature also increases the rate of the reactions that break down rocks. The result is a decrease in CO2 in the atmosphere and a weakening of the greenhouse effect. A weakened greenhouse effect cools the planet and stabilizes the temperature. Similarly, a decrease in temperature reduces evaporation and slows the removal of CO2 from the atmosphere, strengthening the greenhouse effect and counteracting the decrease in temperature.
In combination, the greenhouse effect and the carbon cycle have regulated Earth’s temperature for billions of years. However, this does not mean that the temperature has always been about the same as it is today. Hundreds of millions of years ago, for example, the CO2 content of the atmosphere was much higher than it is now, and the dinosaurs enjoyed a tropical climate with trees in Antarctica.
There have also been large excursions away from stability. Some of the most dramatic are known as the Snowball Earth episodes of about 700-650 million years ago, when the Earth became almost entirely frozen over. With the planet covered in ice, sunlight was reflected back into space by the bright surface instead of being absorbed, leading to further cooling. The carbon cycle and the greenhouse effect provided the eventual escape from the snowball phase, as volcanic outgassing released CO2 into the atmosphere while the absence of evaporation and rainfall kept it there.
Intense volcanic activity has also released massive amounts of CO2 into the atmosphere, at rates much faster than the rest of the carbon cycle can keep up with. Climate change caused by one of these episodes was responsible for the largest mass extinction in Earth’s history, about 250 million years ago. We’ll learn more about this event in the chapter on geological and biological coevolution. Today, the release of CO2 from the burning of fossil fuels again threatens the stability of Earth’s climate.
Key Concepts and Summary
Earth’s atmosphere contains a small fraction of greenhouse gases such as water vapor, carbon dioxide, nitrous oxide, and methane. These gases absorb and reradiate infrared radiation (heat) that would otherwise escape to space, warming Earth by about 30°C and making it habitable. Over long timescales, the CO2 content of Earth’s atmosphere is regulated by the inorganic carbon cycle, in which CO2 in the atmosphere is dissolved in rainwater which then erodes rocks. Sediments from the rocks are carried to the oceans, combining again with CO2 to form new carbonate rocks. The conveyer belt of plate tectonics carries these rocks to subduction zones where they are melted, releasing CO2 to be outgassed by volcanoes. The greenhouse effect and the carbon cycle work together in a negative feedback loop to stabilize Earth’s temperature.
Review Questions
- What is the compositions of Earth’s atmosphere? What are the primary greenhouse gases?
- How do greenhouse gases interact with light from the Sun to warm the Earth?
- How do the greenhouse effect and the carbon cycle work together to stabilize Earth’s temperature?
- How does plate tectonics contribute to Earth’s habitability?
- Can a planet without plate tectonics be habitable?
The atmosphere and greenhouse effect sections of this chapter are remixed from Introduction to Earth Science, Second Edition and Physical Geology under a CC BY-NC-SA 4.0 license.
gases in an atmosphere that trap heat, raising the surface temperature of a planet
a process in which information about the output of a system is routed back as an input to modify the actions being taken by the system

