9 Chapter 9 – Introduction to the Nematodes
General Shape and Structure of Endoparasitic Nematodes
Nematodes are relatively small, mostly dioecious, non-segmented worms that generally lack any sort of well-de- veloped external structures for locomotion. Many of the animal parasitic species possess external cuticular structures that enable them to move and maintain their position in the host and—depending on the host—this could include the gut, mesenteries, sub-cutaneous tissues, or other organs. The external structures of parasitic nematodes that enable them to detect their environment include amphids on the anterior end, deirids (also called cervical papillae) near the level of the nerve ring, phasmids near the tail, and various kinds of sensory sensillae. As far as is known, no animal parasitic nematodes have eye spots, and only a few nematodes that live in marine intertidal interstitial environments have eye spots. Some nematodes have complex lips surrounding the mouth (Figure 1) and these lips facilitate feeding. The lips and associated structures posterior to the mouth may enable the nematode to attach to the host intestine. Not all nematodes have all of these structures and different combinations of characters are used for identification and classification into different groups. Examples of species that attach firmly in the small intestine are species of family Ancylostomidae (Figure 2) which, among others, includes the hookworms (genus Ancylostoma). Anisakids are also known to attach to the submucosal layer of the gastrointestinal tract of their hosts. This includes various species in the genera Anasakis, Terranova, and Pseudo- terranova. These nematodes usually use marine mammals as their definitive hosts.

Figure 1. Scanning electron micrograph of the anterior end of a species of Paraspidodera from Ctenomys in Bolivia showing 3 large lips with sensory papillae, also known as sensillae (plural) or sensillum (singular) on each lip. Source: S. L. Gardner. License: CC BY 4.0.

Figure 2. Image of anterior end of Ancylostoma ctenomyos, a par- asite of rodents of the genus Ctenomys from the eastern lowlands of Bolivia. The stoma with the cutting teeth and plates are clearly visible. The villi of the small intestine are pulled into the stoma and the teeth abrade the villi. Blood then is pumped into the intestine from the abraded villi via the esophagus. Source: S. L. Gardner. License: CC BY 4.0.
On the posterior end of nematodes, males of some groups, such as species of the order Strongylida (Figures 3–5), have a well-developed and complex apparatus called the copulatory bursa that is used to grasp the female to facilitate mat- ing. Other nematodes, such as species of Physaloptera, Oxyurida, and Filarioidea have various combinations of papillae (sensillae) and cuticular ornamentations that serve a similar purpose.
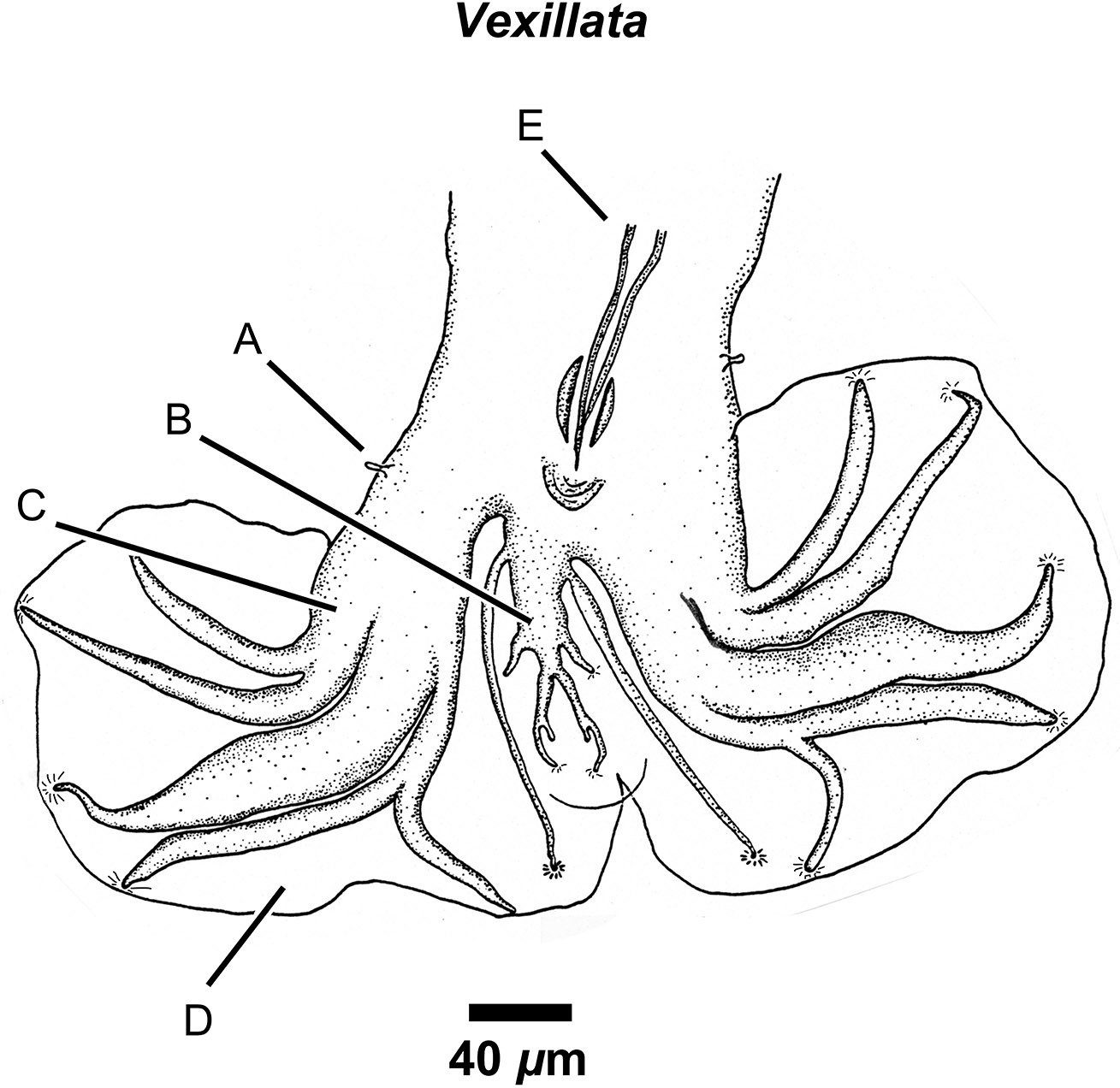
Figure 3. Drawing of the posterior end showing a ventral view of the copulatory bursa of Vexillata armandae (order Strongylida, family Ornithostrongylidae), a parasite of the coarse haired pocket mouse (Chaetodipus hispidus). Labels: A) Papillae (ray 0); B) Dorsal ray; C) Lateral rays in the 2-1-2 arrangement; D) Vellum (thin cuticular membrane) of bursa; E) Setacous spicules retracted. Source: S. L. Gardner. License: CC BY 4.0.
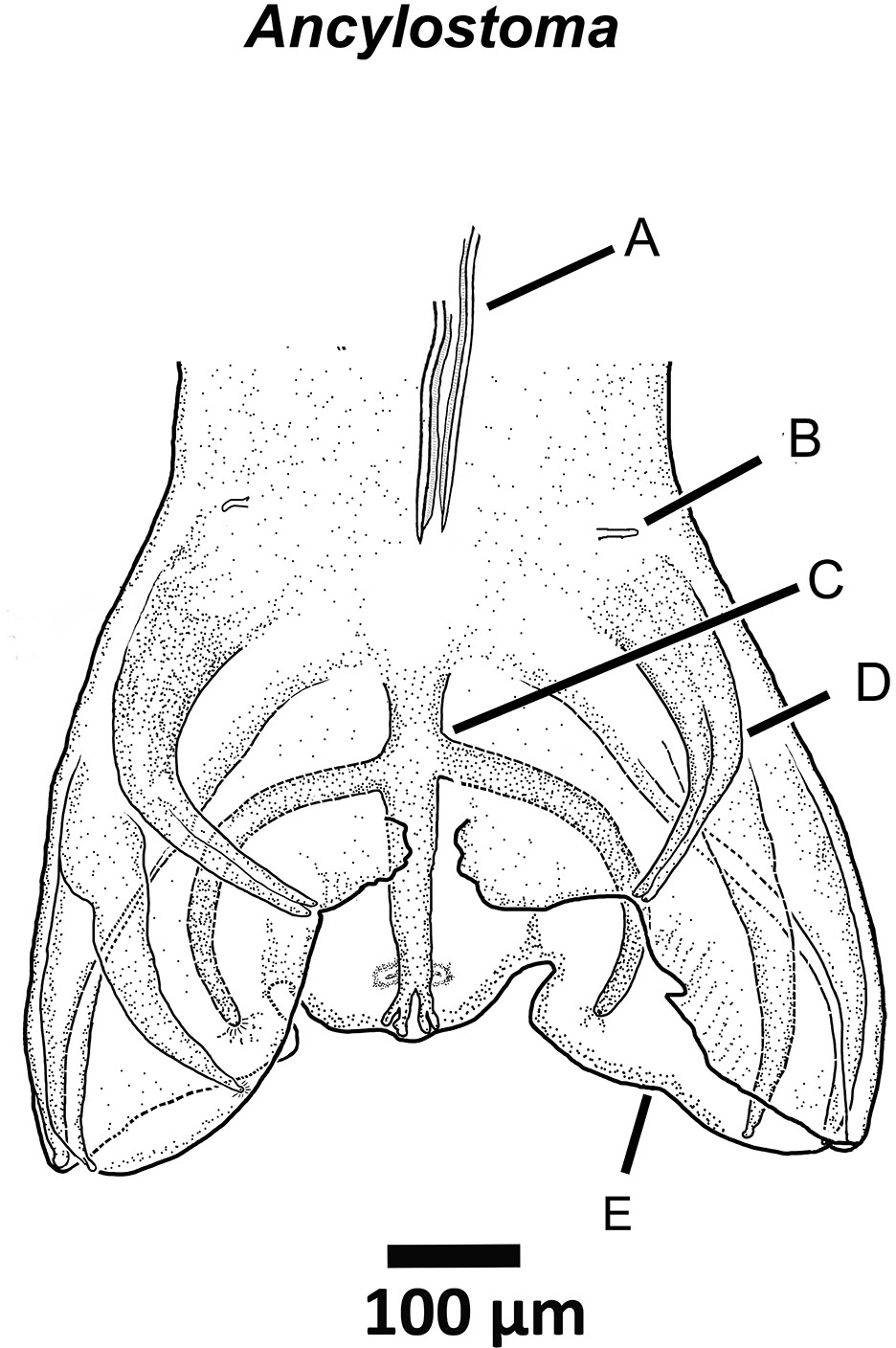
Figure 4. Drawing of the posterior end of a species of Ancylostoma ctenomyos a parasite of rodents of the genus Ctenomys in lowland Bolivia. Labels: A) Posterior ends of the spicules; B) Ray 0 or pre-bursal ray; C) Dorsal ray; D) Lateral rays; E) Vellum of bursa. Source: S. L. Gardner. License: CC BY 4.0.
Depending on the species, the body of parasitic nematodes usually has a greater diameter (when viewed in transverse section) near the middle of the body of the animal relative to the anterior end. All nematodes have a round shape when viewed from either end of a transverse section through mid-body (Figures 5A–D). The body wall of a nematode is covered with a flexible acellular cuticle that is mostly translucent and is secreted by a cellular hypodermis. The circular or round nature of the transverse sections is why these animals are sometimes called roundworms.
The external cuticle of the nematode may be smooth, have longitudinal striations, or have well-developed wing-like structures called alae (= wings; Latin) or ridges that are situated on the lateral surfaces of the body (Figure 5D). Some species have well-developed lateral alae near the anterior end where they are called cervical alae. The tiny wings, or alae, can also run the entire length of the body on the lateral cuticle of the body, in which case they are termed lateral alae, and if only on the posterior, they are called caudal alae. It is thought by some researchers that the alae are utilized by nem- atodes to orient themselves somehow in the host, and there is conclusive evidence that the alae are species-specific and can be used in identifying and classifying the nematodes.
The cuticle of nematodes can be extremely thin and fragile, or it can be thick and extremely strong and even very resistant to digestion, as shown in those species that live in the acid environment like that found in the stomach of a carnivore. For example, filarioid nematodes have a very thin cuticle. They occur in the tissues of vertebrates and are able to exist only within these osmotically balanced habitats and if they are removed and placed in water, they usually quickly explode due to osmotic pressures.
Nematodes are sometimes casually called pseudocoelomates because in most species that have been studied, their body cavity does not appear to be completely lined with cells derived from embryonic mesoderm. All coelomate animals that are termed eucoelomates—or true coelomates—have a peritoneum that lines both the body cavity and the internal organs and the peritoneum is a layer of tissue that is derived from embryonic mesoderm. Most nematodes do not have an obvious or visible lining of the body cavity, although, as Armand Maggenti (1981, p. 10) pointed out: “Nematodes have a well-de- veloped body cavity filled with fluid and with some evidence of mesodermal lining, if one considers the muscle sheath as mesoderm and the epidermal layer around the gonads and the basal lamella of the intestine as being of mesodermal origin.”
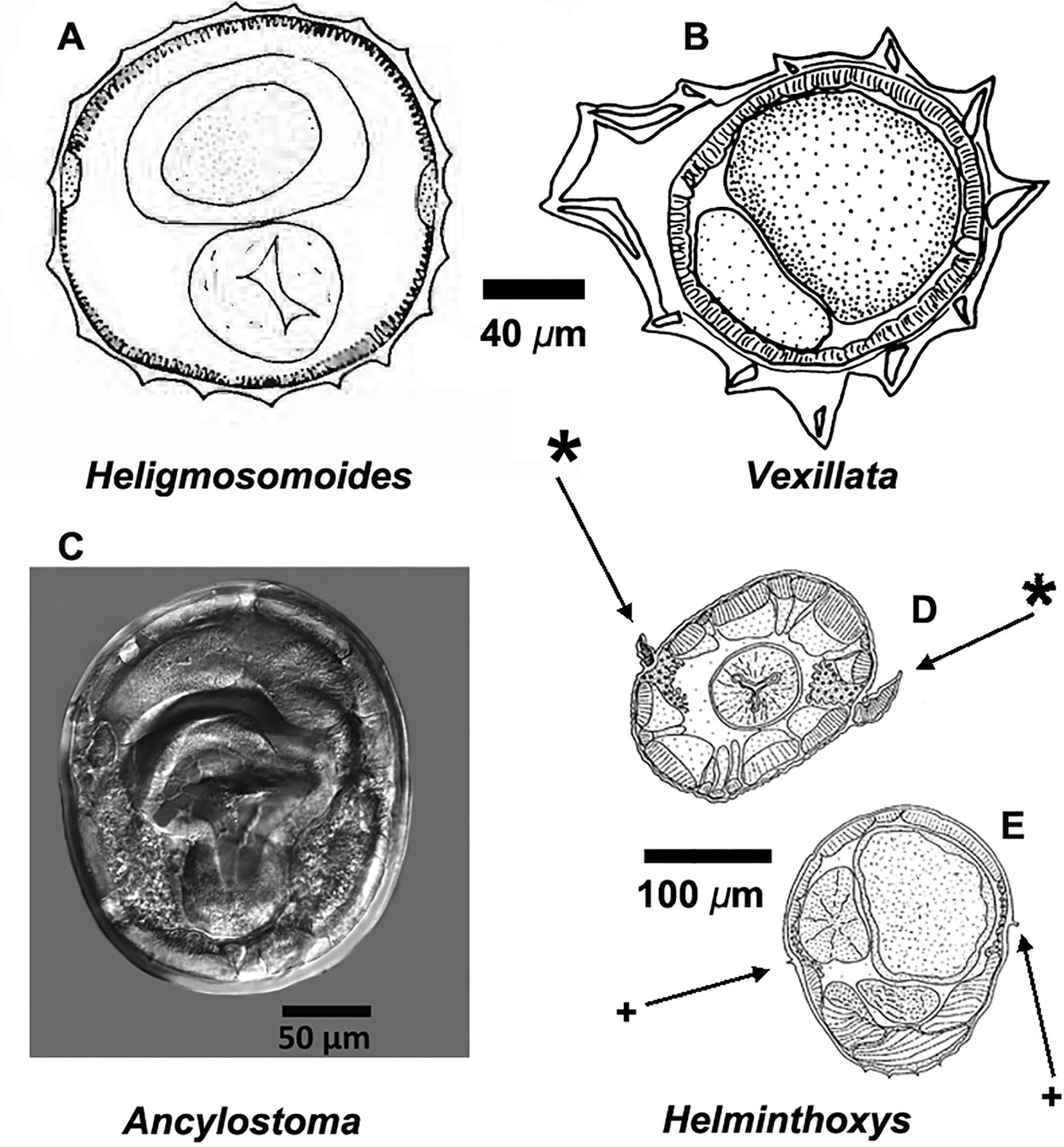
Figure 5. Drawings and photographs of transverse sections from 3 species of superfamily Strongyloidea (Figures A–C), including: A) Transverse section at midbody of Heligmosomoides thomomyos showing the lack of cuticular aretes within the cuticle; B) Transverse section at midbody of a species of Vexillata showing the well-developed carene and well-developed cuticular aretes; C) Digital image of a transverse section of Ancylostoma ctenomyos showing a relatively smooth cuticle; D and E) Transverse sections through a species of Hel- minthoxys (order Oxyurida) with D) Cephalic alae (*) via a cut through the area of the esophagus. E) Transverse cut through the posterior part of the nematode showing the small lateral alae (+). Source: S. L. Gardner. License: CC BY 4.0.
Another unique feature of all nematodes is the fact that they have no circular muscles. Therefore, movement is accomplished by contraction and relaxation of longitudinal muscles in apposition, or antagonistic to, their hydrostatic skeleton. The nematode moves as the muscles contract and the cuticle flexes thus enabling nematodes to writhe around, moving through the organs and tissues of their hosts, and in some cases, into the external environment. Nematodes maintain their form in a way analogous to a water balloon because their body fluids are under a positive pressure in the hydrocoel relative to their environment.
Individuals of most animal parasitic species of nematodes have a complete digestive tract with an anterior stoma lined with cuticle, followed by a pharynx, then a tri-radiate esophagus (Figure 5D) that can be muscular (Figure 6) or glandular in form, or the esophagus may have a combination of both muscular and glandular sections as in some species of the superfamily Filarioidea, and others. The tubular intestine (Figure 6F) is usually a single cell in thickness and is lined on the body cavity side with a thin collagen-like material. Internally, the single layers of cells are lined completely with microvilli (Grassé, 1965; Maggenti, 1981; 1991a). The gastrointestinal tube extends from the esophagus to the anus, or cloaca, with some species possessing out-pouched cecae or diverticulae near the esophageal end (Figure 7).
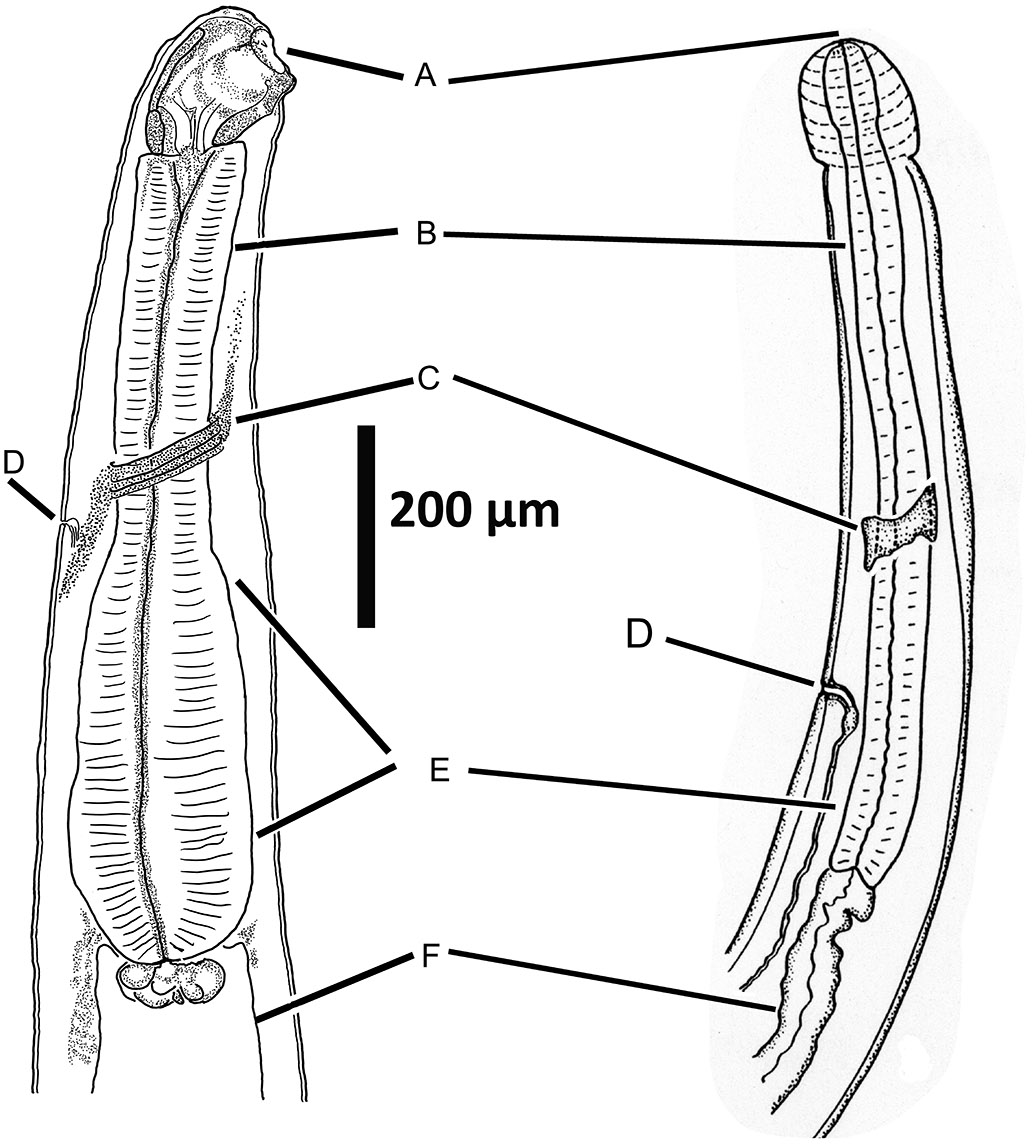
Figure 6. Anterior end of 2 strongylid nematodes showing homol- ogous structures of the anterior end. A) Mouth; B) Anterior end of muscular esophagus; C) Nerve ring, also called the circumesophageal commissure; D) Excretory pore; E) Base of esophagus; F) Intestine. Source: S. L. Gardner. License: CC BY 4.0.
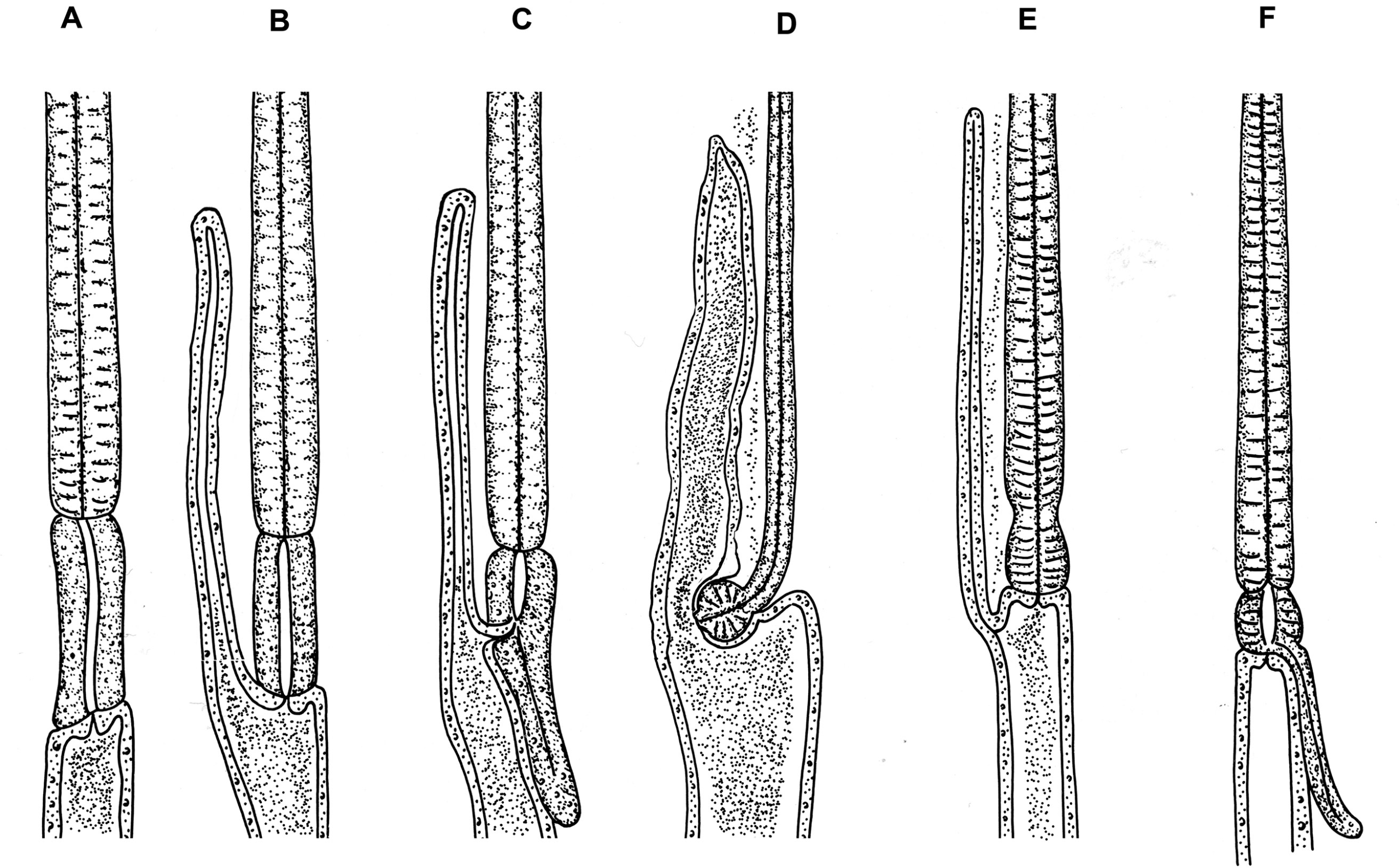
Figure 7. Intestinal cecae or diverticulae that may occur in nematodes of the order Ascaridida. A) Anasakis; B) Porrocaecum; C) Contracaeum; D) Dujardinia; E) Aguticaecum; F) Raphidascaris. Source: Adapted from Maggenti, 1981; Yamaguti, 1961. License: CC BY 4.0.
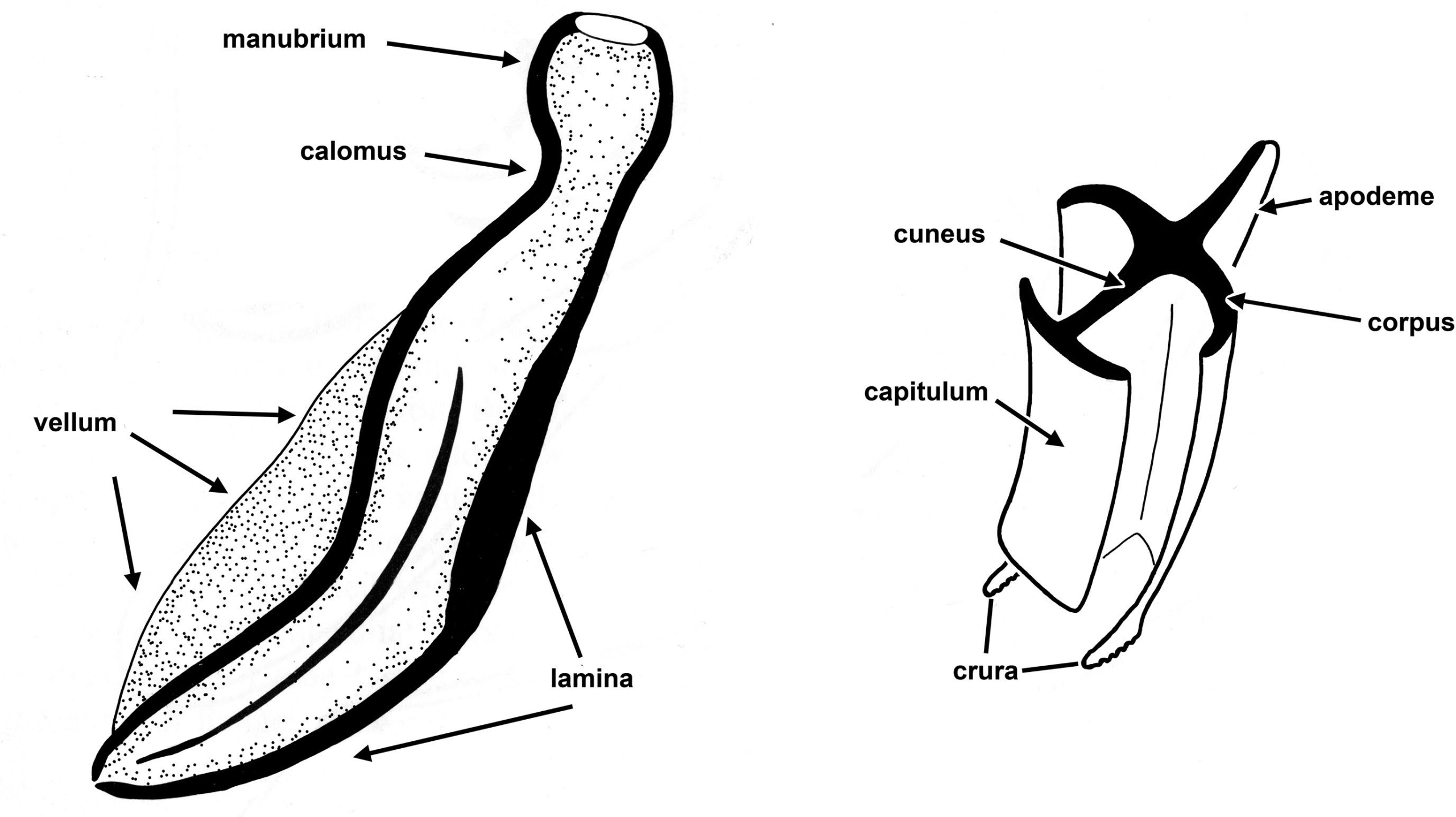
Figure 8. A) Spicule, showing major parts. B) Gubernaculum, transverse section showing how the spicule can slide through the opening between the capitulum and the corpus. Source: Adapted from Maggenti, 1981. License: CC BY 4.0.
Most nematodes are sexually dimorphic with definite male and female individuals, and they are usually dioecious, meaning that they have to mate to produce viable eggs. In those species that are dioecious, males usually have a pair of cuticularized spicules that are used to assist in the transfer of sperm to the females (Figure 8A).
There are some pinworms in which the males have no spicule at all, such as species of Aspiculuris. Many male nem- atodes also have a gubernaculum (Figure 8B) that serves to guide the spicules during copulation. Some species are her- maphroditic and in these cases the nematode produces both sperm and ova from the ovotestis of the same individual at different times during their life stage, or ontogenetic development (Maggenti, 1981).
A major synapomorphy (also called a shared-derived character) for the Nemata is the presence of non-contractile, myo-neural processes that extend from the contractile portion of the muscle cell to the neural junctions of the dorsal or lateral nerve cords (Figure 9). Figure 9 shows the contractile portion of the muscle below and the neural part with the nucleus above with a laterally extending neural process that extends to the nerve cord.
Nematodes vary greatly in size and the diameter of most species is usually much less than 2 mm, even in the longest of the long nematodes such as Placentanema gigantissima, which is a 9 m-long parasitic nematode that lives in the reproductive tract of whales. The thickest ones, or those individuals with the greatest body width of all nematodes known so far, is the giant kidney worm, Dioctophyma renale. This nematode lives in mustelids, such as minks, badgers, and weasels, and has a diameter of up to 15 mm with a length of more than 1 m. The eggs of nematodes are also very similar in size with eggs of most species ranging from 50–100 μm- long by 20–50 μm-wide.
External Covering: The Cuticle
An example of diversity in shape and structure of the cuti- cle is that of the complex cuticular aretes found in most species of the superfamily Trichostrongyloidea that are parasitic in the small intestines of many species of vertebrates, especially mammals, in which the exocuticle is modified into a series of cuticular aretes called the synlophe. In these forms, it is thought that the ridges running down the length of the body of the nematode are used in maintaining their position in the intestine of their hosts (Figure 10). In the latter part of the 1960s and 1970s, an evolutionary schema of nematodes classified in the superfamily Trichostrongyloidea was developed by Marie Claude Durette-Desset and Alain Chabaud in the Laboratoire des vers, Muséum national d’Histoire naturelle in Paris, France (currently named Laboratoirede biologie parasitaire, protistologie, helminthologie). They worked out the biogeographical and morphological evolution of these nematodes using a combination of the structure of the synlophe (Figure 10) and the rays of the bursa (Figure 3) that allowed an understanding of the evolutionary relationships of one of the most speciose groups of parasitic nematodes (Durette-Desset, 1971).
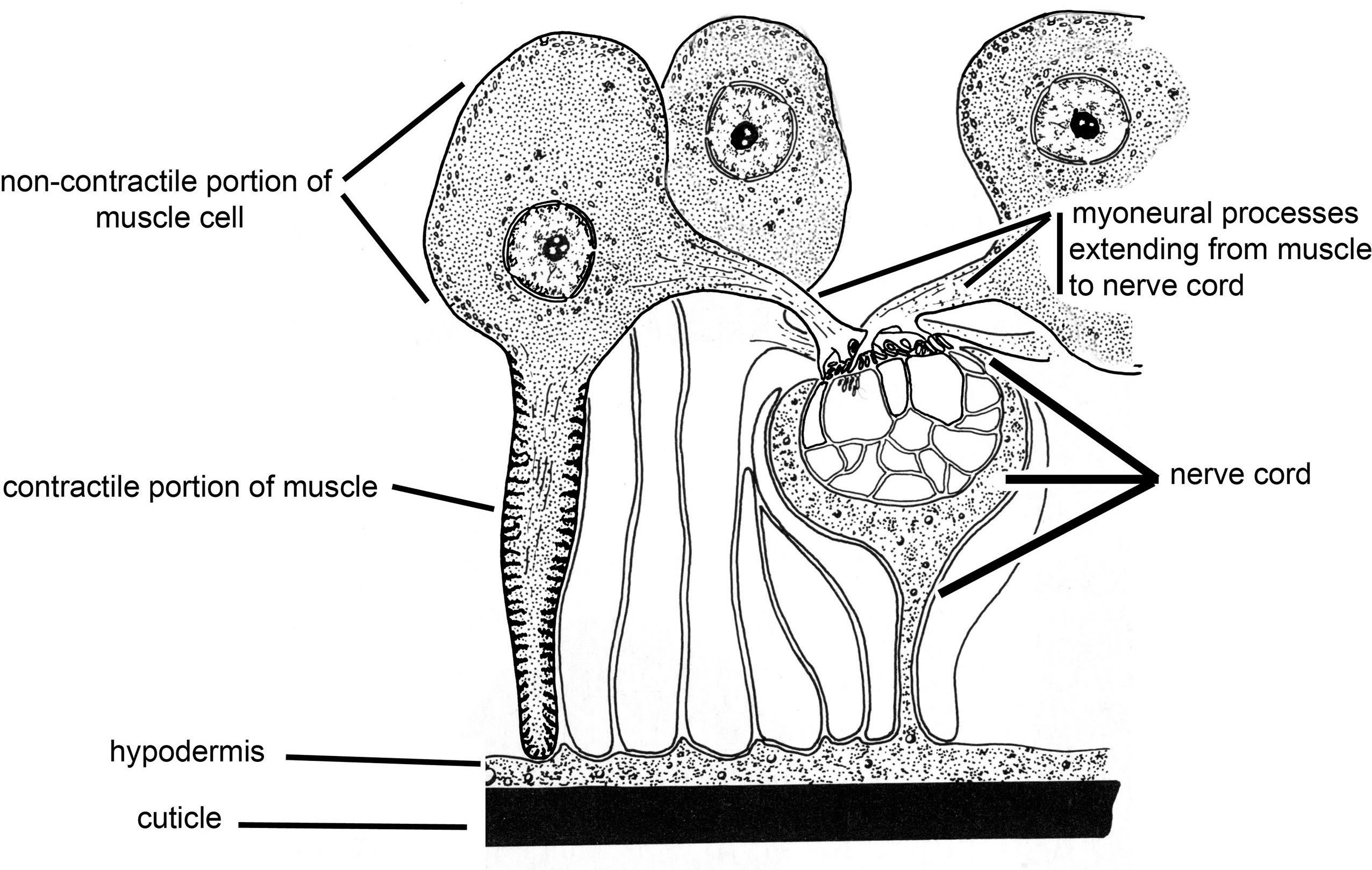
Figure 9. Schematic of the contractile and non-contractile portions of the muscle cells seen in the Nemata. Source: Adapted from Maggenti, 1981. License: CC BY 4.0.
The external cuticle may be smooth without alae or external lines, or the cuticle may have complex rings. In the posterior part near the cloaca of many male nematodes (in the family Physalopteridae, superfamily Filarioidea, and others), and in addition to sensory papillae or sensillae, there may be a rough area of the external cuticle called the area rugosa, or the rough area. A hypothesis about why this is found in males is that the rough cuticle may enable the male to locate and attach to the female more easily for mating purposes. Some nematodes have an exocuticle (external cuticle) composed of serrated ridges or bumps, or the cuticle may be very smooth. Some groups, such as species comprising the superfamilies Heterakoidea and Subuluroidea, possess cuticularized suckers situated anterior to the cloaca that are surrounded by sensory papillae and evidently enables the male to find and attach to the female in the intestinal tract of the host (Figure 11).
Host Range and Diversity of the Nemata
All species of vertebrates and many species of beetles examined by scientists thus far serve as hosts for at least 1 species of parasitic nematode. Some nematodes have a very narrow host range, surviving and reproducing successfully only in a single species of host, or perhaps in a phylogenetically- or ecologically-related group of species. Other nematodes show a wide host range, being much more likely to jump from one suitable host to another during opportune times during their life history, exhibiting what is termed ecological fitting (Janzen, 1985; see also Brant and Gardner, 2000; Brooks et al., 2019).
Within a single free-living animal, myriad habitats may be occupied by nematodes. Organs and tissues of a mammal have different hormone levels, different levels of pH, various levels of exposure or isolation from the immune system, and more. To illustrate the diversity of habitats in a single mammal host, for example in humans, Trichinella species can occur as juveniles, encysted in various muscles like the diaphragm or the tongue, Strongyloides species may be found in the mucosa of the intestine or other tissues, Ascaris may be found migrating in blood and lungs, juveniles called microfilariae of filarioid nematodes may be found in blood or lymph, or adult Ancylostoma, Necator, and Ascaris species may be found in the small- and large intestines. Individuals of these same species, as well as Onchocerca, Loa, and Wuchereria, can occur as juveniles in muscle or connective tissues, and as adults in mesenteries and subcutaneous tissues, and Enterobius vermicularis may be found in the large intestine and cecum.
Figure 10. Transverse sections of Vexillata armandae. A) Section through esophagus of male; B) Section through esophagus of female; C) Midbody of male; D) Midbody of female; E) Posterior 1/4 of body of male through the spicules; F) Posterior 1/4 of body of female through eggs in the uterus; G) Posterior 1/16 of body of male through the spicules just anterior to the cloaca; H) Posterior section of female at level of ovijector infundibulum I) Posterior section of female at level of ovijector vestibule. Source: S. L. Gardner. License: CC BY 4.0.
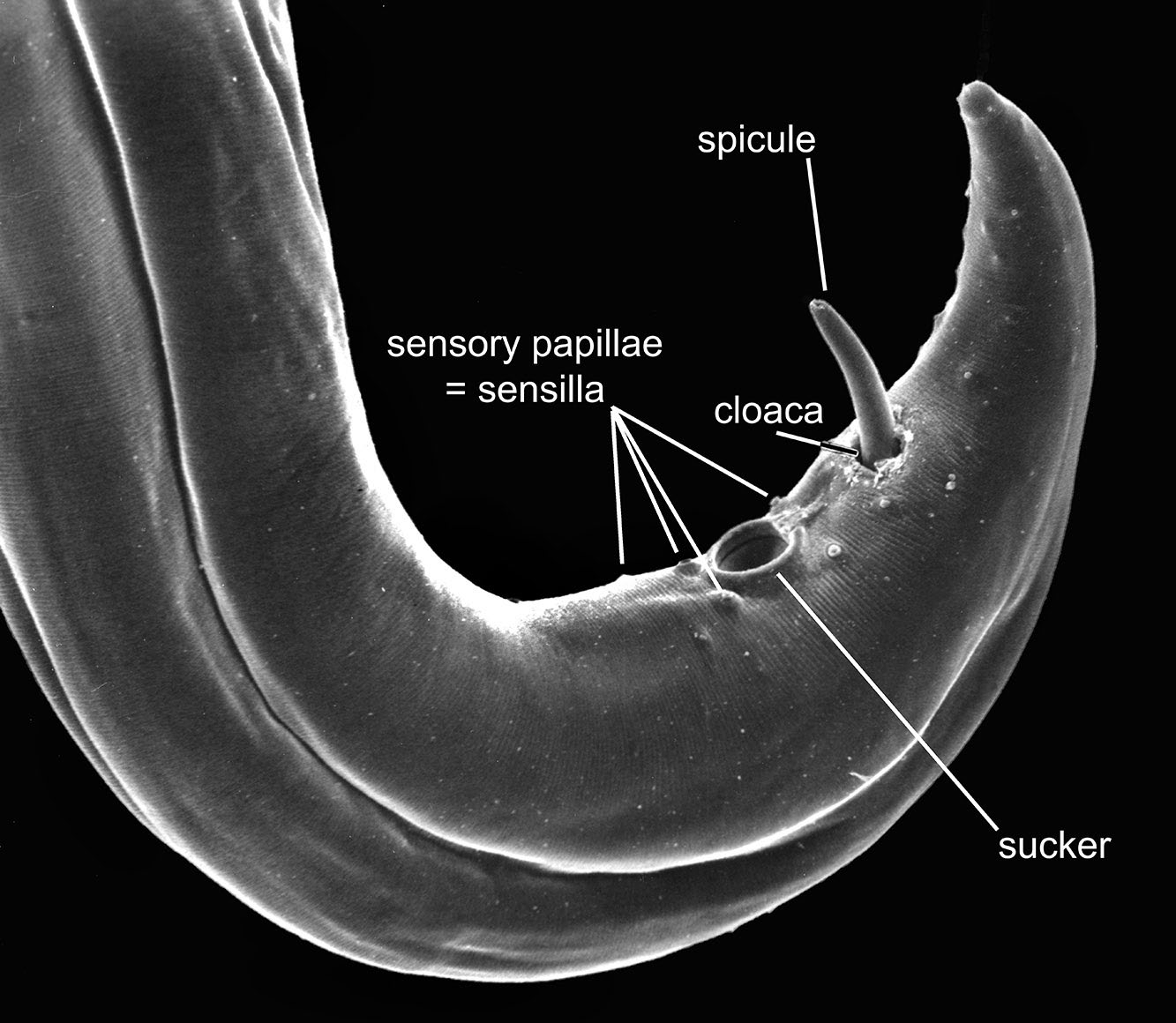
Figure 11. Posterior end (tail) of a male Paraspidodera showing the relatively smooth cuticle, a sucker just anterior to the opening of the cloaca, and 1 spicule protruding from the cloaca. Source: S. L. Gardner. License: CC BY 4.0.
In the early part of the 20th century, Nathaniel Cobb (1915) recognized that nematodes are extremely biodiverse. He was well acquainted with animal parasitic nematodes, and his familiarity with the Nemata led him to estimate that well over 80,000 species of nematodes would eventually be found parasitizing vertebrates alone. Falling short of Cobb’s estimate, to date only about 20,000 species of parasitic nematodes have been described from all species of 48,000-plus recognized species of vertebrates. The natural history, development, and transmission parameters of more than 550 species of animal nematodes are well known (Anderson, 2000). As parasites of vertebrates, Anderson (2000) estimated that there were about 2,300 described genera distributed among 256 families comprising about 33% of all nematode genera. If each of the approximately 6,526 known species of mammals (as of July 2023; see https://mammaldiversity.org) were each infected with only 2 species of nematodes with narrow host range, there would be expected to be a minimum of 13,052 species of parasitic nematodes only in the class Mammalia.
Pinworms, which are nematodes of the order Oxyurida (Figure 12), have a relatively narrow host-range and it is well known that almost all species of rodents, lagomorphs, and primates have their own species of pinworm. Both recent and historical studies have shown that pinworm nematodes exhibit relatively narrow host range, and many have been shown to have cospeciated with their primate and rodent hosts (Hugot, 1999). Currently, a total of about 900 species of pinworms have been described, with vertebrates hosting about 500 species and invertebrate species hosting only about 400. Because pinworms tend to have a narrow host range, their numbers correlate approximately with the number of host species there are. By way of example, pinworms that are parasitic in arthropods are classified in the superfamily Thelastomatoidea. There are currently about 4,500 described species of cockroaches with more than 20,000 to 30,000 additional species expected to be eventually described (Ghosh, 2017). There are around 17,000 species of millipedes known with more than 60,000 species expected yet to be described. If each species of cockroach and millipede harbors its own species of pinworm, huge numbers of thelastomatoid pinworms will eventually be described from just these 2 arthropod groups alone.
The greatest diversity in the superfamily Oxyuroidea of vertebrates expected to be found in the future may result from examination of the catfishes of the Amazon basin (Rodrigues et al., 2020). During studies of the catfishes of the Amazon basin, Rodrigues (personal communication, 2020) stated that every fish examined was infected with oxyurids.
It is estimated that approximately 138 species of nematodes have been reported from humans (Crompton, 1999), with 32 to 36 being host-specific.
Large numbers of species of Oxyuroidea are also expected to be described from Neotropical rodents of the family Muridae. As of 2022, only around 8 species of pinworms have been described from Neotropical murids, while there may be 400–800 undescribed species of Oxyuroidea, given that 1–2 new species of oxyuroid nematode are found in each new species of rodent examined.
Classification
In this book, the use of the phylum name Nemata (= thread; German) for the nematodes partially follows Hodda’s (2022) work, as well as the older work by Maggenti (1981; 1991a) who considered Chitwood’s (Chitwood and Chitwood, 1977) emendation of the name Nemates to Nemata a correct and robust move because this followed the Pearse system for nomenclatural endings (Pearse, 1936). Maggenti (1981) points out that the old phylum name Nematoda is a leftover class level name from previously discarded classifications that were developed when the nematodes were considered to be a class in a larger group called the now-superceded phylum Aschelminthes. However, not all biologists who study nematodes adhere to this system; see, for example, the contrasts in older classifications in the work by Libbie Hyman (1953), the massive work by the French zoologist Pierre-Paul Grassé (1965), and the book General Nematology by Maggenti (1981) for the variations.
Hodda’s (2022) work includes an explanation about why the upper-level classification of the Nemata is confusing and difficult. Maggenti (1981) and Anderson (2000) are generally followed for the higher order names for the animal parasitic nematodes and Hodda (2022) is generally followed for the names lower in the classification; however, in this work, no great effort has been made to synchronize the classifications with Hodda (2022) and others since changes are being made daily as more data from genomic sequencing efforts roll into the databases holding information on the Nemata.
All told, above the level of the order, confusion reigns relative to the classification and systematic arrangement of the nematodes, although Hodda’s (2022) efforts should bring some measure of stability to the classification of the group. Maggenti (1981) is usually followed in the upper levels of the classification and the phylogeny presented by Anderson (2000; see Figure 12) provides the main groups; however, there are some points of agreement between Maggenti’s and Anderson’s with Hodda’s more recent (2022) classification. The classes recognized in this book include the Secernen- tea and the Adenophorea (Figure 13). Recent work shows that these groups are mostly substantiated both in morphological and molecular analyses although competing phylogenetic hypotheses and associated classifications have also been proposed (Adamson, 1989; Dorris et al., 1999; Blaxter et al., 1998; Brooks and McLennan, 1991; Anderson, 2000; Hodda, 2022).
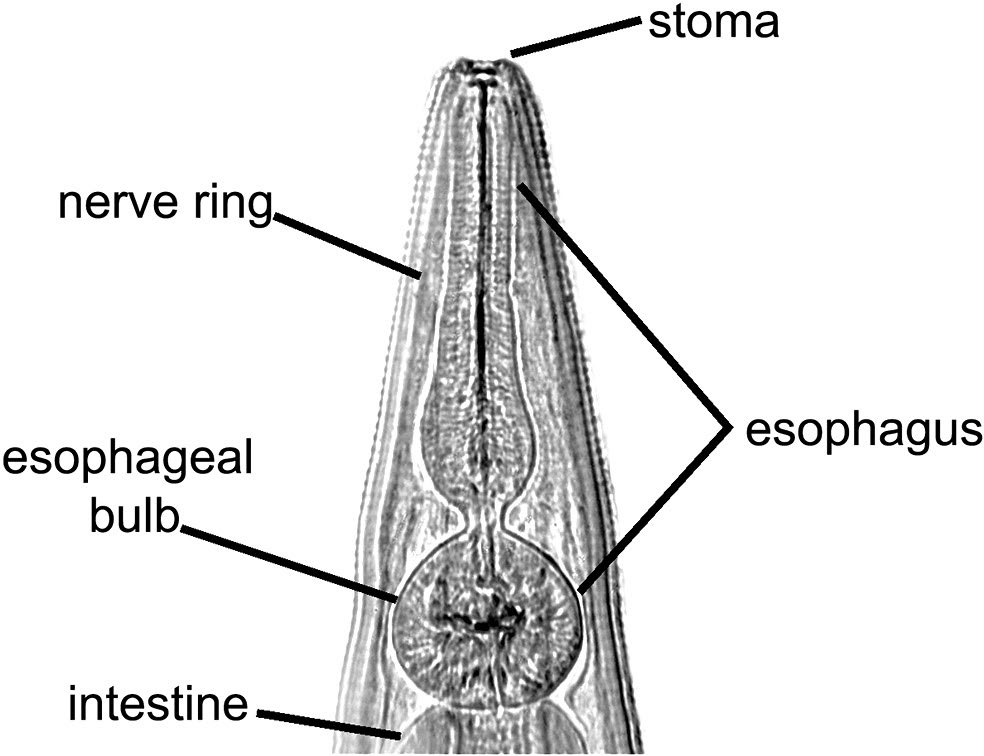
Figure 12. Anterior end of Didelphoxyuris, a pinworm nematode of South American marsupials, showing: A) The small stoma at the anterior end, followed by the well-developed muscular esophagus with a large posterior bulb. The esophagus acts as a muscular pumping organ, pumping food into the intestine. Source: S. L. Gardner. License: CC BY 4.0.
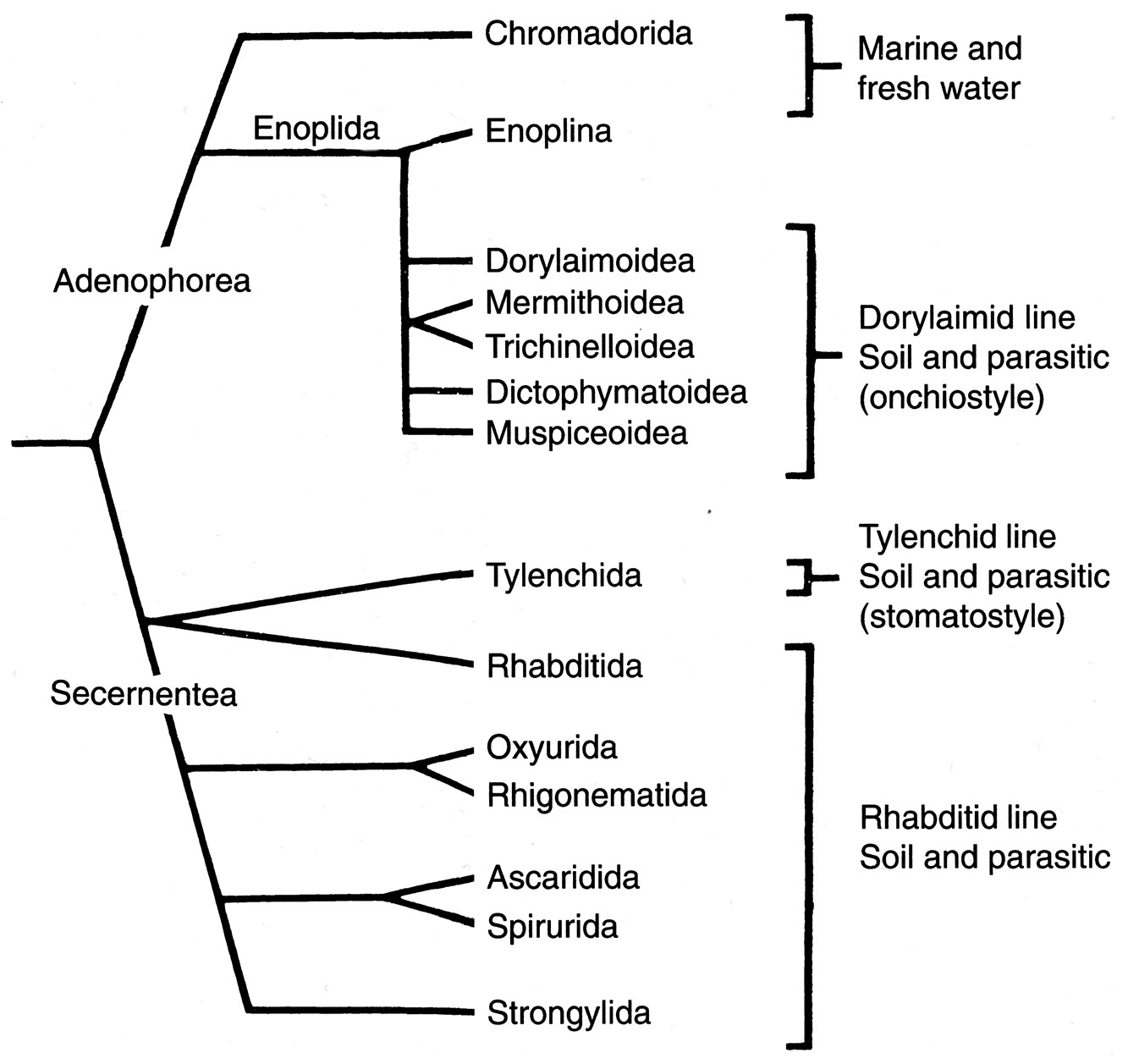
Figure 13. Phylogenetic hypothesis of main groups of the phylum Nemata. Sources: Adapted from Anderson, 2000; Maggenti, 1991. License: CC BY-NC-SA 4.0.
Relation to Other Animal Groups
One analysis grouped the nematodes, gastrotrichs, priapulids, kinorhynchs, and the loriciferans into a superphylum group called the Cycloneuralia based on the circular shape of the nerve ring that loops around the esophagus in most groups and functions as the central part of the nervous system in these animals (Nielsen et al., 1996). Other studies have shown that the Nemata share a common ancestor with the Nematomorpha (see Zrzavy, 1998). Mayer and Whitington (2009) and Nielsen (2012) show that the Nemata share a common ancestor with the Nematomorpha in a different superphylum designation called the Nematoida.
Ancient History of Nemata
The oldest written account of the giant intestinal nematode Ascaris lumbricoides in humans dates to approximately 4,750 years ago, from China. In this work, foods to avoid and a description of the symptoms of humans infected with these worms was accurately given (Hoeppli, 1959; Maggenti, 1981). In the area of the Nile River Valley, early Egyptian physicians first recorded the presence of both Ascaris and the guinea worm, also called the fire worm, Dracunculus medinensis, in an ancient papyrus manuscript written by Egyptian physicians around 3,550 years ago, which was obtained and translated by the Egyptologist Georg Ebers in 1872 (see Chit- wood and Chitwood, 1977; Maggenti, 1981). About 2,400 years ago, Hippocrates first wrote about nematodes infecting other animals besides humans when he recorded his finding of pinworm nematodes of horses. In the 13th century, Albertus Magnus and Demetrios Pepagomenos recorded nematodes from falcons (cited in Rausch, 1983; see also Chitwood and Chitwood, 1977).
Physical evidence of nematodes date from much earlier than the written accounts. Eggs of the pinworm of humans, Enterobius vermicularis, and the whipworm of humans, Trichuris trichuria, occur in coprolites dated to about 7,000 years old from Peru, and eggs of the human-specific hookworm, Ancylostoma duodenale, have been reported from cop- rolites dated to around 7,230 years old, collected from caves in eastern Brazil (Araújo et al., 2008). Eggs of Ascaris lum- bricoides have been positively identified from coprolites of human origin dated to about 28,000 years old from caves in France, but these are the only occurrence of a record this old (Bouchet et al., 1996). The dearth of other nematodes from human remains older than about 7,000 years appears to be due to the fact that organic material comprising the coprolites themselves do not preserve well enough to last that long (Karl J. Reinhard, personal communication, 2022).
Trace or Fossil History
The only fully fossil nematodes that are known thus far are insect parasitic or plant parasitic forms that occur very rarely in amber inclusions (Poinar et al., 1994). One pinworm egg was found in a fossilized fecal pellet from a cynodont around 250 million years old (Hugot et al., 2014). Because there are no fossil records of nematodes of Cambrian or Precambrian ages, estimates of the age of the Nemata have been only speculation up to the present time and without fossils, it is difficult to calibrate molecular clocks for the nematodes. An estimate of the time of divergence of the nematodes from the rest of the animal groups appears to be about 1,177 ± 79 mil- lion years (Wang et al., 1999).
Most authors consider the ultimate origin of nematodes to be from a marine ancestor (Maggenti, 1981; Malhakov, 1994). The tri-radiate esophagus and the tubular body indicate an initial possible primarily sedentary existence of the ancestral forms of the Nemata, with the posterior end attached to the substrate and the anterior end freely encountering the marine environment from all sides, thus the somewhat radial symmetry may be secondarily-derived from selective advantage of the animal experiencing the environment from all sides simultaneously (Armand Maggenti, personal communication, 1992).
Original Textbook Chapter 48 Authors:
Scott L. Gardner
Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States; and School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, Nebraska, United States slg@unl.edu
Revisions: Reordered the chapters to match class organization. Removed the section on Nematode Section Organization since most of that material will be covered in the laboratory portion of the course.
Literature Cited
Adamson, M. 1989. Constraints in the evolution of life histories in zooparasitic Nematoda. In R. C. Ko, ed. Current Concepts in Parasitology. Hong Kong University Press, Hong Kong, p. 221–253.
Anderson, R. C. 2000. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd edition. CAB International, Wallingford, United Kingdom, 650 p.
Araújo, A., K. J. Reinhard, K. Ruiz, and S. L. Gardner. 2008.Parasites as probes for prehistoric human migrations? Trends in Parasitology 24: 102–116. doi: 10.1016/j.pt.2007.11.007
Blaxter, M. L., P. De Ley, J. R. Garey, X. Liu, et al. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392: 71–75. doi: 10.1038/32160
Bouchet, F., D. Baffier, M. Girard, P. Morel, et al. 1996.Paléoparasitologie en contexte pléistocène: Premières observations à la Grande Grotte d’Arcy-sur-Cure (Yonne), France. Comptes rendus de l’Académie des sciences, Série 3; Sciences de la vie 319: 147–151.
Brabec, J., E. D. Salomaki, M. Kolísko, T. Scholz, et al. 2023. The evolution of endoparasitism and complex life cycles in parasitic platyhelminths. Current Biology 33: 4,269–4,275. doi: 10.1016/j.cub.2023.08.064
Brant, S. V., and S. L. Gardner. 2000. Phylogeny of species of the genus Litomosoides (Nemata: Onchocercidae) evidence of rampant host-switching. Journal of Parasitology 86: 545–554. doi: 10.1645/0022-3395(2000)086[0545:POSOTG]2.0.CO;2
Brooks, D. R., and D. A. McLennan. 1991. Phylogeny, Ecology, and Evolution: A Research Program in Comparative Biology. University of Chicago Press, Chicago, Illinois, United States, 441 p.
Brooks, D. R., E. P. Hoberg, and W. A. Boeger. 2019. The Stockholm Paradigm: Climate Change and Emerging Disease. University of Chicago Press, Chicago, Illinois, United States, 409 p.
Chitwood, B. G., and M. B. Chitwood. 1977. Introduction to Nematology. University Park Press. Baltimore, Maryland, United States, 334 p.
Cobb, N. A. 1915. Nematodes and their relationships. In Yearbook of Department of Agriculture for 1914. United States Government Printing Office, Washington DC, United States, p. 457–490.
Crompton, D. W. T. 1999. How much human helminthiasis is there in the world? Journal of Parasitology 85: 397–403.
Dorris, M., P. De Ley, and M. L. Blaxter. 1999. Molecular analysis of nematode diversity and the evolution of parasitism. Parasitology Today 15: 188–193.
Durette-Desset, M. C. 1971. Essai de classification des Nématodes Heligmosomes. Corrélations avec la paleobiogéographie des hôtes 69: Mémoires du Muséum national d’Histoire naturelle, Série A: Zoologie, Paris, France, 126 p.
Ghosh, J. 2017. A study on the occurrence of pinworms in the hindgut of Periplaneta americana. Journal of Parasitic Diseases 41: 1,153–1,157. doi: 10.1007/s12639-017-0952-0
Grassé, P. P. 1965. Traité de Zoologie: Anatomie, Systématique, Biologie, Tome IV, Fascicule II: Némathelminthes (Nématodes), and Fascicule III: Nématodes, Gordiacés, Rotifères, Gastrotriches, Kinorhynques, 1,497 p.
Hodda, M. 2022. Phylum Nematoda: Trends in species descriptions, the documentation of diversity, systematics, and the species concept. Zootaxa 1668: 265–293. doi: 10.11646/ zootaxa.5114.1.2
Hugot, J.-P. 1999. Primates and their pinworm parasites: The Cameron hypothesis revisited. Systematic Biology 48: 523–546. doi: 10.1080/106351599260120
Hugot, J.-P., S. L. Gardner, V. Borba, P. Araújo, et al. 2014. Discovery of a 240 million-year-old oxyurid nematode parasite egg sheds light on the early origin of nematode parasitism in vertebrates. Parasites and Vectors 7: 486. doi: 10.1186/s13071-014-0486-6
Hyman, L. H. 1940–1959. The Invertebrates, Volumes 1–5. McGraw Hill, New York, New York, United States.
Janzen, D. H. 1985. Coevolution as a process: What parasites of plants and animals do not have in common. In K. C. Kim, ed. Coevolution of Parasitic Arthropods and Mammals. Wiley, New York, New York, United States.
Maggenti, A. R. 1981. General Nematology. Springer-Verlag, New York, New York, United States, 372 p.
Maggenti, A. R. 1991a. Nemata: Higher classification. In W. R. Nickle, ed. Manual of Agricultural Nematology. Dekker, New York, New York, United States, p. 147–187.
Maggenti, A. R. 1991b. General nematode morphology. In W. R. Nickle, ed. Manual of Agricultural Nematology. Dekker, New York, New York, United States, p. 3–46.
Malakhov, V. V. 1994. Nematodes: Structure, Development, Classification, and Phylogeny. D. Hope, ed.; G. V. Bentz, transl. Smithsonian Institution Press, Washington, DC, United States, 286 p.
Mayer, G., and P. M. Whitington. 2009. Velvet worm development links myriapods with chelicerates. Proceedings of the Royal Society B: Biological Sciences 276: 3,571–3,579. doi: 10.1098/rspb.2009.0950
Nielsen, C. 2012. Animal Evolution: Interrelationships of the Living Phyla. Oxford University Press, Oxford, United Kingdom, 402 p.
Nielsen, C., N. Scharff, and J. D. Eibye. 1996. Cladistic analyses of the animal kingdom. Biological Journal of the Linnean Society 57: 385–410. doi: 10.1111/j.1095-8312.1996.tb01857.x
Pearse, A. S. 1936. Zoological Names: A List of Phyla, Classes, and Orders. Duke University Press, Durham, North Carolina, United States, 24 p.
Poinar, G. O., A. Acra, and F. Acra. 1994. Earliest fossil nematode (Mermithidae) in cretaceous Lebanese amber. Fundamental and Applied Nematology 17: 475–477.
Rausch, R. L. 1983. The biology of avian parasites: Helminths. In D. S. Farner, J. R. King, and K. C. Parkes, eds. Avian Biology, Volume VII. Academic Press, New York, New York, United States, p. 367–442.
Rodrigues, A. R. O., Y. Wilkens, F. T. V. Melo, S. L. Gardner, et al. 2020. Oxyuricassis ekstromi n. sp. (Oxyurida: Pharyngodonidae) from Lasiancistrus saetiger (Siluriformes: Loricariidae) from the eastern Amazon. Journal of Parasitology 106: 611–615. doi: 10.1645/19-5
Wang, D., S. Kumar, and B. Hedges. 1999. Divergence time estimates for the early history of animal phyla and the origin of plants, animals, and fungi. Proceedings of the Royal Society of London, Series B 266: 163–171. doi: 10.1098/ rspb.1999.0617
Wilson, D. E., and D. M. Reeder, eds. 2005. Mammal Species of the World: A Taxonomic and Geographic Reference, Volumes 1 and 2, 3rd edition. Johns Hopkins University Press, Baltimore, Maryland, United States, 2,142 p.
Zrzavy, J., S. Mihulka, P. Kepka, A. Bezdek, et al. 1998. Phylogeny of the Metazoa based on morphological and 18S ribosomal DNA evidence. Cladistics 14: 249–285. doi: 10.1111/j.1096-0031.1998.tb00338
Supplemental Reading
Brooks, D. R, and D. A. McLennan. 1993. Parascript: Parasites and the Language of Evolution. Smithsonian Institution Press, Washington, DC, United States.
Durette-Desset, M. C. 1985. Trichostrongyloid nematodes and their vertebrate hosts: Reconstruction of the phylogeny of a parasitic group. Advances in Parasitology 24: 239–306.
Durette-Desset, M. C., and A. G. Chabaud. 1981. Nouvel essai de classification des Nématodes: Trichostrongyloidea. Annales de parasitologie humaine et comparée 56: 297–312.
Morand, S., P. Legendre, S. L. Gardner, and J.-P. Hugot. 1996. Body size evolution of oxyurid (Nematoda) parasites: The role of hosts. Oecologia 107: 274–282. doi: 10.1007/ BF00327912
Musser, G. G., and M. D. Carleton. 1993. Family Muridae. In D. E. Wilson and D. M. Reeder, eds. Mammal Species of the World: A Taxonomic and Geographic Reference. Smithsonian Institution Press, Washington, DC, United States, p. 501–755.
Rentz, D. 2014. A Guide to the Cockroaches of Australia. CSIRO Publishing, Clayton South, Victoria, Australia, 326 p.

