3 Chapter 3 Introduction to the Platyhelminths
Introduction to Platyhelminths
The phylum Platyhelminthes includes the cestodes, trematodes, and monogeneans (which are classified in one treatment as monopisthocotylids and polyopisthocotylids; see Brabec et al., 2023). Most worms classified into Platyhelminthes have bodies that are dorsoventrally flattened, so they are sometimes referred to as flatworms. They may be leaf shaped or oval and some are more or less rounded, and some, such as tapeworms, grow to large sizes. For example, parasitic flatworms range in size from those that are nearly microscopic such as species of Gyrodactylus that live on the gills of fishes and have a maximum body length of less than 500 µm to the giant cestodes of whales like Tetragonoporus calyptocephalus that live in the intestine of sperm whales and can attain a length of more than 30 m. Adult flatworms lack a coelom and are called acoelomate but they do possess a well-developed mesoderm, which becomes parenchyma, reproductive organs, and musculature.
Platyhelminths also are bilaterally symmetrical and thus have a definite anterior end with associated sensory and motor nerve elements. The nervous system is elaborate in many species enabling them to live in a wide variety of habitats, including inside other animals, lakes and streams, moist terrestrial environments, and ocean sediments worldwide. The bodies of other animals are quite hospitable to some platyhelminths, so they are commonly parasitic. Platyhelminths can even serve as hosts for other platyhelminths; for example, some cercariae (free-swimming transmission stages of trematodes that have left their snail host) can and do penetrate planarians and encyst, developing to metacercariae that are the stages that are infective to the next host in a complex life cycle (Fried and Rosa-Brunet, 1991).
A plesiomorphic condition of platyhelminth physiology is their apparent inability to synthesize fatty acids and sterols de novo, which may help explain why platyhelminths are most often symbiotic with other organisms, either as commensals or parasites (Meyer and Meyer, 1972). Free-living acoel turbellarians, sometimes considered illustrative of ancestral platyhelminths, also seem to lack this ability, indicating that this loss occurred before the parasitic forms evolved from the basal species of the group. Being soft bodied, platyhelminths have left a relatively poor fossil record, but some evidence suggests they have been on Earth for eons, for instance, fossil tracks from a slab of Permian siltstone have been interpreted as those of a land planarian.
General Platyhelminth Morphology
The outer covering of these animals is called the tegument and the structure and function varies among species in the major taxonomic groups. Generally speaking, turbellarians and some free-living stages of trematodes and cestodes have a tegument composed of ciliated epithelium (Figure 1), which in some cases is their primary mode of locomotion. This epithelium consists of a single layer of cells and contains many glandular cells and ducts from subtegumental glands. Sensory nerve endings are abundant in the tegument. In some platy- helminths, cells that produce adhesive secretions are paired with those that produce releasing secretions; the combination is known as a duo-gland adhesive system.
Adult trematodes and cestodes have no external cilia except in larval stages such as miracidia (miracidum, singular) in trematodes and coracidia (coracidium, singular) in some
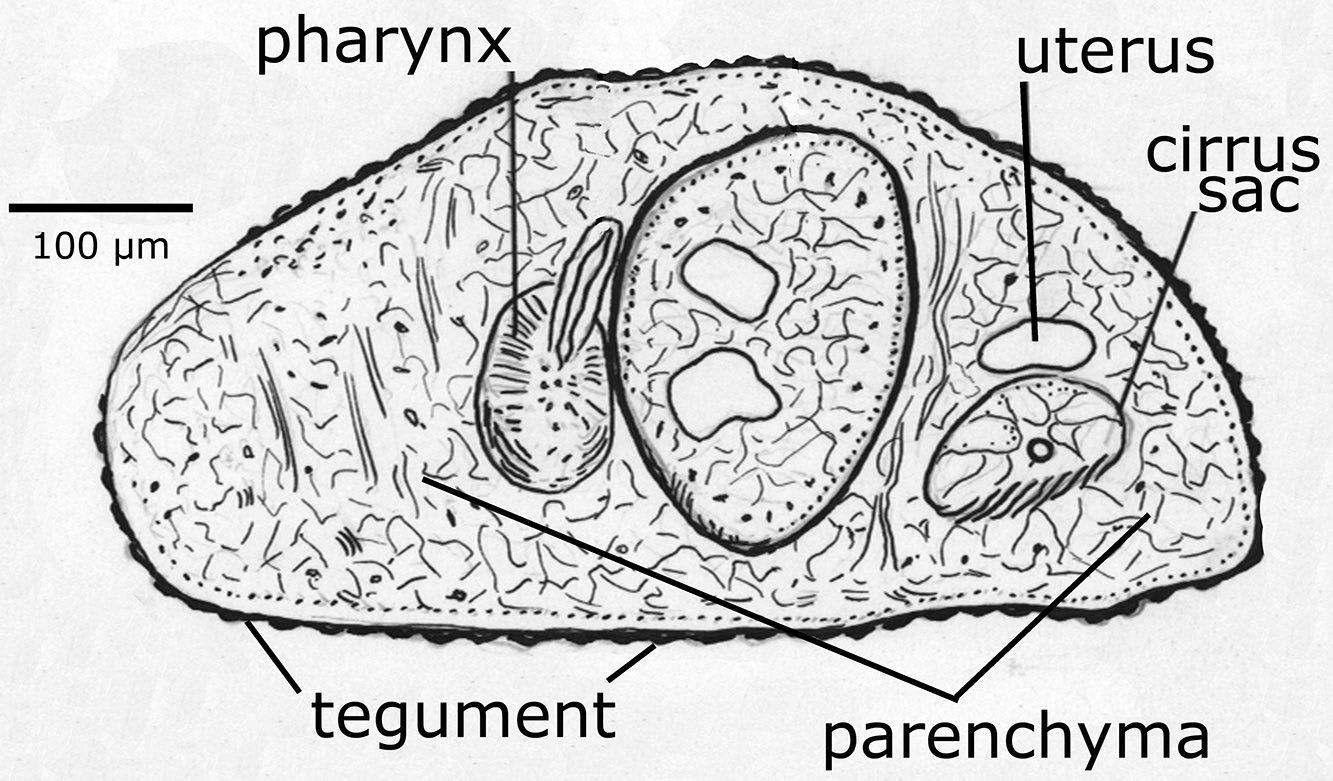
Figure 1. Cross section of a parasitic trematode showing the lack of ciliated epithelium. Source: H. W. Manter. License: CC BY.
cestodes. During metamorphosis of these parasitic forms, the larval epidermis is replaced by a syncytial adult tegument (see Figure 2). The syncytium is a continuous cellular matrix without the normal intercellular membranes and with nuclei of which are in cell bodies (cytons) located beneath a superficial muscle layer. Thus, the name Neodermata (neo = new, derma = skin; Greek) has been used in classifications at the subphylum level to distinguish such worms from free-living species that retain the ciliated epithelium as adults. Most of a platyhelminth’s body is made up of parenchyma, a loosely arranged mass of fibers and cells of several types. Some of these cells are secretory, others store food or waste products, and still others have huge mitochondria and function in regeneration. The internal organs are so intimately embedded in the parenchyma that dissecting them free of the surrounding tissue is nearly impossible. The bulk of the parenchyma probably is composed of myocytons (non-contractile part of muscle cells).
Muscle fibers course through the parenchyma. Contractile portions of muscle fibers are rarely striated and are usu- ally arranged in 1 or 2 longitudinal layers near the body surface, just beneath the syncytial epidermal layer. Circular and dorsoventral fibers also occur.
The nervous system of platyhelminths is a ladder type, with paired ganglia near the anterior end, nerves running anteriad to sensory or holdfast organs, and longitudinal nerve trunks extending posteriad subterminal to the body (meaning, to nearly the end of the body). The number of trunks varies, but most trunks are lateral and are connected by transverse commissures. The nervous system and morphological variation could be used for classification and determination of species, but the techniques of staining and study are quite difficult to master, so not many parasitologists use these characteristics for diagnostic purposes.
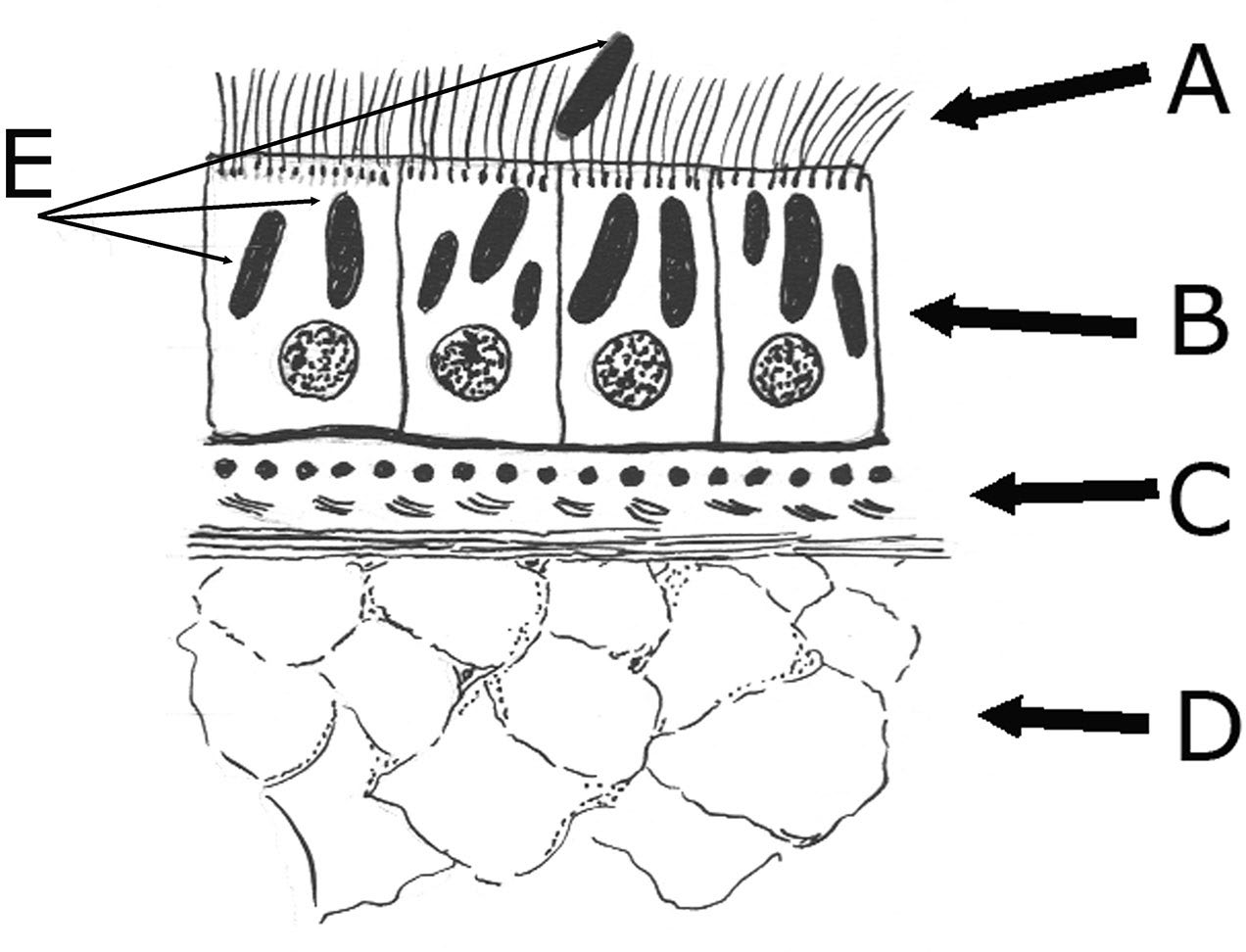
Figure 2. Example section through the body of a turbellarian flat- worm. Ciliated tegument (A), epithelial or tegumentary cells (B), circular, longitudinal, and diagonal muscles (C), parenchyma (D), and rhabdites (E). Source: H. W. Manter. License: CC BY.
Sensory elements are abundant and may be distributed in a variety of patterns, depending on the species. Tactile cells, chemoreceptors, eye spots, and statocysts have been reported from platyhelminths and these have received vari- ous levels of study.
The digestive system of parasitic platyhelminths is typically a blind sac, also called a cecum, although a few trematodes, such as the species that live in the intestines of bats, the morphologically minuscule Anenterotrema spp., have only a mouth, and perhaps a pharynx, but no gut at all. Most platyhelminths with a digestive system have a mouth near their anterior end, and most trematodes have a muscular pharynx with which they suck in food through the mouth. The gut varies from a simple bi-lobed/bifurcating sac to a highly branched tube, but only rarely do trematodes have an anus. Digestion is primarily extracellular, with phagocytosis by intestinal epithelium (gastrodermis), which may contain both secretory and phagocytic cells (Bogitsh, 1993). Undigested wastes are regurgitated back out through the mouth. Relative to this fact, Libbie Hyman (1951, p. 6) said, “The value of an anus cannot be overstated. It permits the animal to feed continuously without waiting for the intestine to be emptied of the previous meal. It also permits a more thorough digestion of food by allowing the food to remain longer in the intestine and by permitting a one-way flow of digestive juices.”
Note that cestodes completely lack a digestive system of any sort during all stages of development (see Figure 3).
The functional unit of the excretory system of almost all platyhelminths are arrays of flame cells, or protonephridia. These single cells are arrayed through the parenchyma and
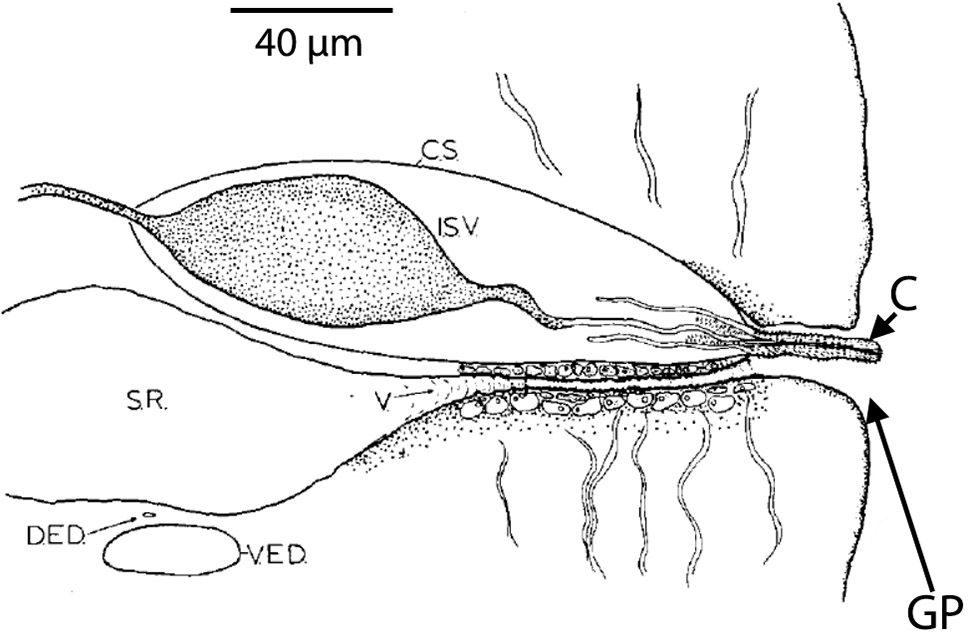
Figure 3. Transverse section through a mature segment of a cestode (tapeworm) showing the dorsal excretory duct (DED) just above the ventral excretory duct (VED). This view is from the posterior end of the worm looking anteriad (meaning, toward the anterior). The seminal receptacle (SR) in this species expands after a very short vagina (V). Dorsal to the vagina is the cirrus sac (CS) with internal seminal vesicle (ISV) shown. The cirrus (C) is shown with minute spines and is shown protruding slightly through the genital pore (GP). In this species the vagina enters the genital pore ventral to the cirrus and cirrus sac. Source: S. L. Gardner, HWML. License: CC BY.
each flame cell comprises a tuft of flagella that extends into a delicate tubule, which may consist of another cell interdigitating with the first (Hertel, 1993; Rohde, 2001). As is the case with the nervous system, ultrastructural studies aimed partly at uncovering characters of evolutionary significance have shown that platyhelminth excretory systems are far more complex than originally thought. Rohde (2001) showed that detailed structure of the flame cell system in various species is correlated more with evolutionary relationship than to habitat in which the animals are living. Protonephridial systems have at least 3 types of flame cells and as many kinds of tubule cells (Rohde, 2001). Excess water, which may contain soluble nitrogenous wastes, is forced into the tubule, which joins with other tubules, eventually to be eliminated through 1 or more excretory pores. Filtration occurs through minute slits formed by rods, or extensions of the cell, collectively called a weir (Old English wer: A fence placed in a stream to catch fish). In parasitic platyhelminths the weir is formed by rods from both the terminal flagellated cell (the cyrtocyte) and a tubule cell and is thus referred to as a 2-cell weir. Because excreta are mainly excess water, this system is often referred to as an osmoregulatory system, with excretion of other wastes considered a secondary function. Some species have an excretory bladder just inside the pore (see Figure 4 to see a line drawing of some platyhelminth structures).
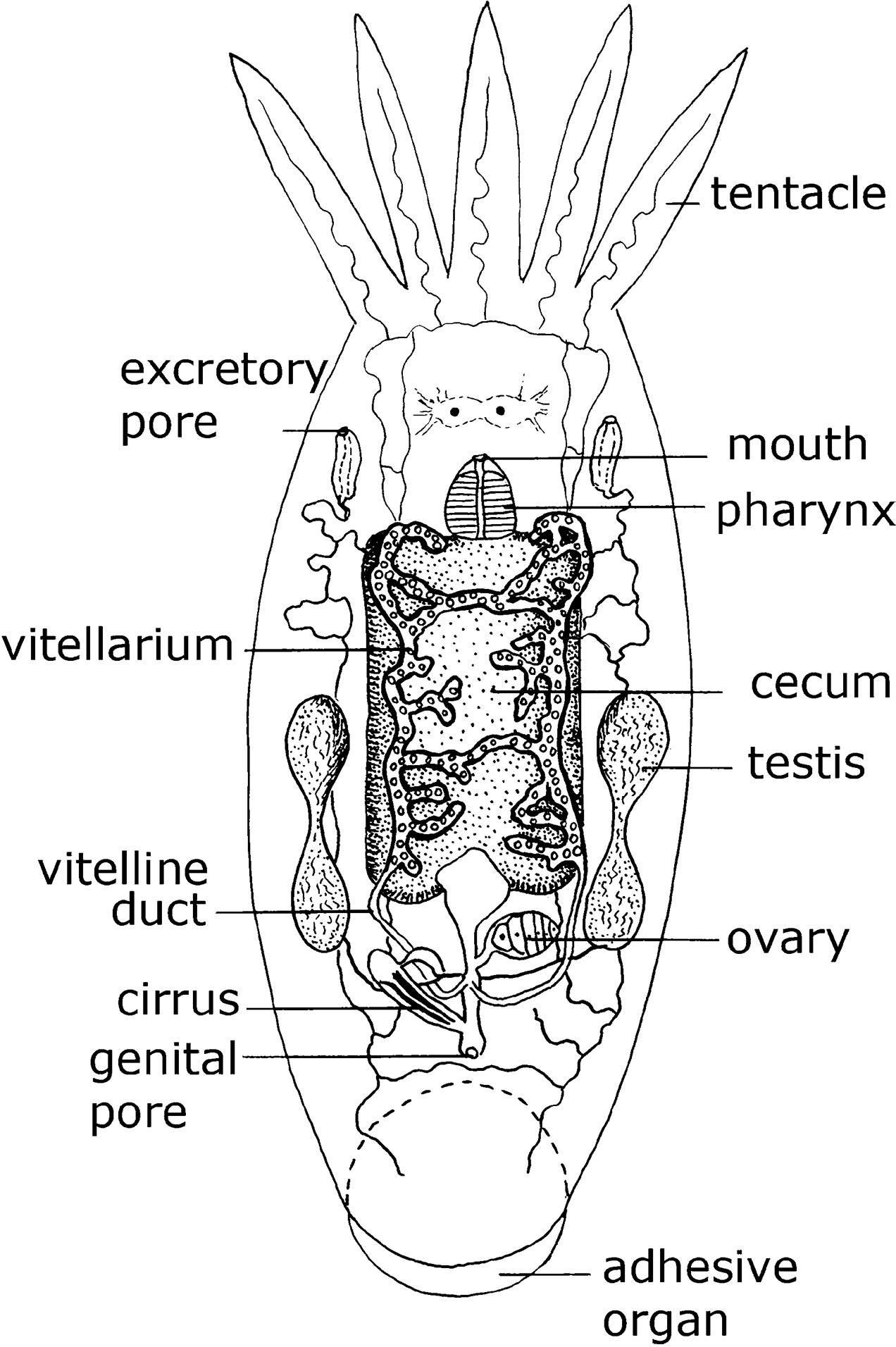 Figure 4. Dorsal view of a marine dwelling flatworm Temnosewellia semperi (Weber, 1890) (phylum Platyhelminthes: order Rhabdocoela: family Temnocephalidae) that normally occurs on the external carapace of freshwater crabs. Source: Adapted from Bresslau and Reisinger, 1933. License: CC BY.
Figure 4. Dorsal view of a marine dwelling flatworm Temnosewellia semperi (Weber, 1890) (phylum Platyhelminthes: order Rhabdocoela: family Temnocephalidae) that normally occurs on the external carapace of freshwater crabs. Source: Adapted from Bresslau and Reisinger, 1933. License: CC BY.
Reproductive systems follow a common basic pattern in all Platyhelminthes. However, extreme variations of this basic pattern are found among different groups. Most species are monoecious (meaning that structures for both sexes are present in a single organism), but a few are dioecious (with separate sexes among individual animals). Because the arrangements of structures in the reproductive systems of platyhelminths are important autapomorphic and synapomorphic characters and include numbers, sizes, shapes, cellular make up, and more, and are used to identify parasites, they are considered in more detail in each specific group. Most hermaphrodites have the ability to fertilize their own eggs, but many do not do so except under exceptional circumstances and cross-fertilization is the norm. Some turbellarians and cestodes have the potential to practice hypodermic impregnation, which is sperm transfer through piercing the body wall with a male organ, the cirrus, and injecting sperm into the parenchyma of the recipient. How sperm find their way into the female system is not known, but the sperm end up in the genital tract of the female part of the worm, whether we know how it happens or not. Most worms, however, deposit sperm directly into the female tract. Larvae usually develop within egg membranes, but a few species are viviparous or ovoviviparous. In species that live as parasites and some turbellarians, egg yolk is supplied by cells other than the ovum that are from the vitelline glands, and eggs are thus ectolecithal. Asexual reproduction is also common in trematodes and a few cestodes.
Introduction to Platyhelminth Systematics
Historically, the phylum has included 4 classes: Turbellaria, Monogenea (also classified as Monopisthocotyla and Polyopisthocotyla; see Brabec et al., 2023), Trematoda (subclass Digenea), and Cestoda, generally corresponding to: Free-living forms, ectoparasitic single-host worms, endoparasitic flukes with 2 or more hosts (1 almost always a mollusc), and tapeworms, respectively. In this book, the focus is on the subphylum Neodermata, particularly the Monogenea, Trematoda (Digenea), and Cestoda. This list of classes implies that the higher classification is settled, but this is not true. Investigations into the phylogenetic relationships of Platyhelminthes is an active area of research in invertebrate biology with many workers attacking evolutionary problems from a variety of directions. The older literature in this area is organized following traditional classifications, for example, see Grassé (1961), Hyman (1951), and for cestodes, the Zoology of Tapeworms (Wardle and McLeod, 1952).
Although parasitic is not necessarily a valid criterion for separating taxa, parasitic platyhelminths have been shown to form a monophyletic group, having been derived from a common ancestral species called the Neodermata. As noted above, this is based on the fact that all known species in this group shed their epidermis at the end of their larval life and when they transition to adults (a general synapomorphy for these worms).
Some phylogenetic studies (Littlewood and Olson, 2001; Brabec et al., 2023) indicate a common ancestor of both Cestoda and species of the proposed class Monopisthocotyla (see Brabec et al., 2023). Morphological or developmental characters such as the nature or origin of the egg yolk, spermiogenesis, body wall musculature, or structure of the excretory organs (especially the flame cells), are used in platyhelminth classification. Molecular characters that have been used include 18S and 28S ribosomal DNA sequences, genes for cytochrome oxidase, NADH dehydrogenase, elongation factor 1-α, and immunochemistry of neurotransmitters (Litvaitis and Rohde, 1999; Mariaux and Olson, 2001; Raikova et al., 2001). Phylogenies based on molecular characters do not always agree with those based on morphology (Littlewood and Bray, 2001) and phylogenies based on molecular characters do not always agree among themselves.
Extant species of flatworms (Metazoa: Acoelomata: Platyhelminthes) represent a lineage of diploblastic metazoa that are considered to show evolutionarily static pictures of the hypothetical ontogenetic stages ultimately showing the development of triploblastic-coelomate (Metazoa: Coelomata) organisms. Most authors agree that the basal extant taxon of the parasitic flatworms is related to species of Stenostomum (subphylum Catenulida), and Stenostomum spp. have been used to root many of the phylogenetic trees that have been developed to examine the evolutionary relationships of the various parasitic flatworms (Ehlers, 1986; Litvaitis and Rohde, 1999; Brooks and McLennan, 1993). The major structural feature dividing catenulid platyhelminths from the rest is the lack of a frontal organ, which is a terminal or subterminal pit with mucoid gland cells and sometimes cilia. Catenulids lack this organ, although some species have lateral pits. Some authors doubt that frontal organs are homologous among the taxa that possess them. Nevertheless, Catenulida appear as basal and as a sister taxon to all remaining Platyhelminthes (except Acoela and Nemertodermatida) in the consensus tree of Littlewood et al. (1999), although it should be recognized that a consensus tree is simply a way to summarize disparate trees and a consensus tree does not represent a phylogeny (Swofford et al., 1996).
While not all scientists agree upon taxon names or hierarchical levels, the classification produced by Brooks and McLennan (1993) was based on their phylogenetic tree and utilizes the full range of rules of classification in the use of superclasses, subsuperclasses, infraclasses, cohorts, and subcohorts in addition to some of the more commonly used terms such as classes and orders. Rohde’s (1996) phylogeny is based both on a small amount of data from 18S ribosomal DNA and on reassessment of structural features, including information on spermiogenesis. This phylogeny differs from that of the morphological phylogeny of Brooks and McLennan (1993) mainly in placement of Temnocephalidea and Udonellidea. The consensus tree provided by Littlewood and Olson (2001) was developed using both morphological and a limited set of molecular data.
The multigene phylogeny of the parasitic flatworms published by Brabec and colleagues (2023) includes phylogenetic trees (shown modified in Figures 5A and 5B) that are based on a more recent assessment than Littlewood and Olson’s (2001). Their work was done to examine hypotheses of the evolution and origin of endoparasitism in the Platyhelminthes
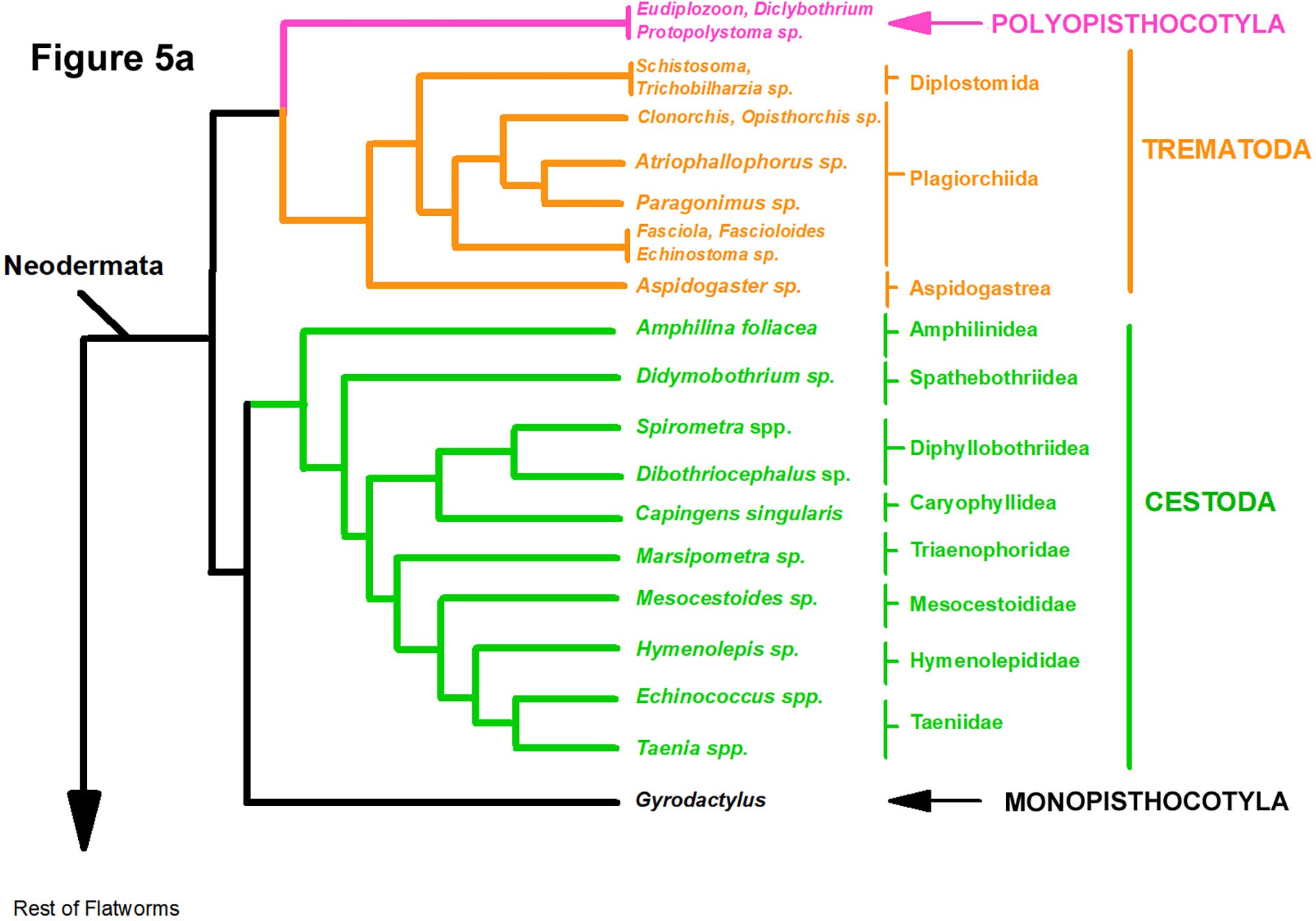
Figure 5A. Phylogeny based on 83 species of helminths of which 51 were parasites. Tree based on Bayesian inference algorithms showing a common ancestor of the Monopisthocotyla and the Cestoda while the Polyopisthocotyla shares a common ancestor with the Trematoda (see Brabec et al., 2023). Source: Adapted from Brabec et al., 2023. License: CC BY.
and included the phylogenetic analyses of 225 genes from 83 taxa (51 of which were parasitic forms). Two equally plausible trees were shown by these authors who used 2 different tree construction algorithms, 1 showing members of the class Monopisthocotyla (ectoparasites) as sister to the class Cestoda (Figure 5A) and the other tree showing species of the class Monopisthocotyla sharing a common ancestor with the trematodes, species of the class Polyopisthocotyla (including Protopolystoma and other genera) and cestodes (Figure 5B). Their main conclusions are:
- The Neodermata includes those flatworms that lose their epidermis upon transitioning from free-living larval forms into sexually reproducing adult forms.
- The mode of living as parasitic flatworms evolved independently in the Neodermata.
- Complex life histories of the cestodes and the trematodes originated independently.
Brabec and colleagues’ (2023) analysis of the origins and diversification of the flatworms represents the tip of the molecular iceberg, hinting that phylogenetic analyses of genomic and proteomic data will eventually become common operations for biologists in the future.
The classification of Platyhelminthes will likely undergo more changes based on new data producing new phylogenies, but the book Interrelationships of the Platyhelminthes edited by Littlewood and Bray (2001) will be the standard reference on platyhelminth systematics for some years to come (Gardner, 2002) even though the conclusions within Littlewood and Bray (2001) have more recently been subjected to rigorous testing by Brabec and colleagues (2023). This is the way science works, hypotheses are produced, tested with new information, and new hypotheses supersede the old ones (Hull, 1988).
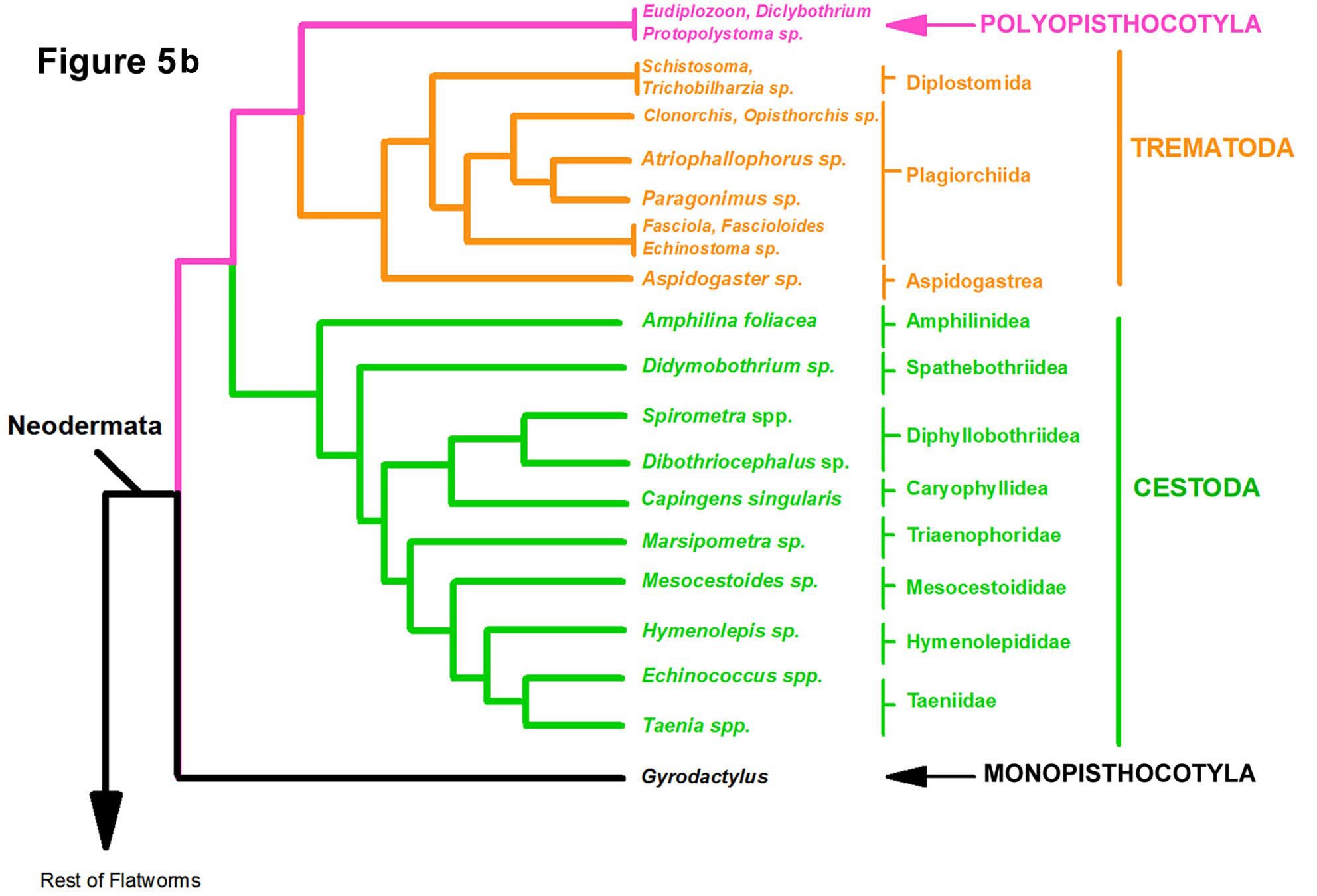
Figure 5B. Phylogeny based on 83 species of helminths of which 51 were parasites. Tree based on the maximum likelihood algorithm showing a common ancestor of the Polyopisthocotyla with the Trematoda and the Monopisthocotyla sharing a common ancestor with the rest of the Neodermata (see Brabec et al., 2023). Source: Adapted from Brabec et al., 2023. License: CC BY.
Note about Placement of Monogenea and Transversotrema in the Book
Note that the Monogenea (lately proposed to be classed as Monopisthocotyla and Polyopisthocotyla by Brabec and colleagues (2023) are covered in Part V of the book (Ectoparasites). Some species of this group live inside their host (endoparasitic) while most species are ectoparasitic. Transvers trema trematodes are also included in the ectoparasite part.
Acknowledgement
This section was partially adapted with permission from Roberts et al. (2014, p. 191–195, 309). The license for this adaptation is CC BY-NC-SA 4.0.
Original Chapter 15 Authors
Larry S. Roberts
Department of Biological Sciences, Texas Tech University, Lubbock, Texas, United States
John J. Janovy, Jr.
School of Biological Sciences, University of Nebraska– Lincoln, Lincoln, Nebraska, United States; and Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States jjanovy1@unl.edu
Steven Nadler
Department of Entomology and Nematology, University of California, Davis, Davis, California, United States sanadler@ucdavis.edu
Scott L. Gardner
Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States; and School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, Nebraska, United States slg@unl.edu
Revisions: Rearranged chapter order in new book. Now chapter 3 to better correspond with laboratory material.
Literature Cited
Bogitsh, B. J. 1993. A comparative review of the flatworm gut with emphasis on the Rhabdocoela and Neodermata. Transactions of the American Microscopical Society 112: 1–9. doi: 10.2307/3226777
Brabec, J., E. D. Salomaki, M. Kolísko, T. Scholz, et al. 2023. The evolution of endoparasitism and complex life cycles in parasitic platyhelminths. Current Biology 33: 4,269–4,275. doi: 10.1016/j.cub.2023.08.064
Bresslau, E., and E. Reisinger. 1933. Plathelminthes. In W. Kuekenthal and T. S. Krumbach, eds. Handbuch der Zoologie B: Allgemeine Einleitung zur Naturgeschichte der Plathelmin- thes 2: 34–51.
Brooks, D. R., and D. A. McLennan. 1993. Parascript: Parasites and the Language of Evolution. Smithsonian Institution Press, Washington, DC, United States, 429 p.
Ehlers, U. 1986. Comments on the phylogenetic system of the Platyhelminthes. Hydrobiologia 132: 1–12. doi: 10.1007/ BF00046222
Fried, B., and L. C. Rosa-Brunet. 1991. Exposure of Dugesia tigrina (Turbellaria) to cercariae of Echinostoma trivolvis and Echinostoma caproni (Trematoda). Journal of Parasitology 77: 113–116. doi: 10.2307/3282568
Gardner, S. L. 2002. Interrelationships of the Platyhelminthes [Book review]. Systematic Biology 51: 192–194. doi: 10.1080/10635150210318
Grassé, P.-P. 1961. Traité de zoologie: Anatomie, systématique, biologie, Tome 4, Fascicule I: Plathelminthes, Mésozoaires, Acanthocéphales, Némertiens. Masson et Cie, Paris, France, 944 p.
Hertel, L. 1993. Excretion and osmoregulation in the flatworms. Transactions of the American Microscopical Society 112: 10–17. doi: 10.2307/3226778
Hull, D. L. 1988. Science as a Process: An Evolutionary Account of the Social and Conceptual Development of Science. University of Chicago Press, Chicago, Illinois, United States, 586 p.
Hyman, L. H. 1951. The Invertebrates, Volume II: Platyhelminthes and Rhynchocoela, the Acoelomate Bilateria. McGraw- Hill, New York, New York, United States.
Littlewood, D. T. J., and R. A. Bray, eds. 2001. Interrelationships of the Platyhelminthes. Taylor and Francis, London, United Kingdom, 356 p.
Littlewood, D. T. J., and P. D. Olson. 2001. Small subunit rDNA and the Platyhelminthes: Signal, noise, conflict, and compromise. In D. T. J. Littlewood and R. A. Bray, eds. Interrelationships of the Platyhelminthes. Taylor and Francis, London, United Kingdom, p. 262–278.
Littlewood, D. T. J., K. Rohde, and K. A. Clough. 1999. The interrelationships of all major groups of Platyhelminthes: Phylogenetic evidence from morphology and molecules. Biological Journal of the Linnean Society 66: 75–114. doi: 10.1111/j.1095-8312.1999.tb01918.x
Litvaitis, M. K., and K. Rohde. 1999. A molecular test of platyhelminth phylogeny: Inferences from partial 28S rDNA sequences. Invertebrate Biology 118: 42–56. doi: 10.2307/3226911
Mariaux, J., and P. D. Olson. 2001. Cestode systematics in the molecular era. In D. T. J. Littlewood and R. A. Bray, eds. Interrelationships of the Platyhelminthes. Taylor and Francis, London, United Kingdom, p. 127–134.
Meyer, F., and H. Meyer. 1972. Loss of fatty acid biosynthesis in flatworms. In H. Van den Bossche, ed. Comparative Bio- chemistry of Parasites. Academic Press, New York, New York, United States, p. 383–393.
Raikova, O. I., M. Reuter, and J.-L. Justine. 2001. Contributions to the phylogeny and systematics of the Acoelomorpha. In D.
T. J. Littlewood and R. A. Bray, eds. Interrelationships of the Platyhelminthes. Taylor and Francis, London, United Kingdom, p. 13–23.
Roberts, L. S., and J. J. Janovy, Jr. 2012. Foundations of Parasitology, 9th edition. McGraw-Hill Higher Education, Boston, Massachusetts, United States, 670 p.
Rohde, K. 1994. The origins of parasitism in the Platyhelminthes. International Journal for Parasitology 24: 1,099–1,115. doi: 10.1016/0020-7519(94)90185-6
Rohde, K. 2001. Protonephridia as phylogenetic characters. In D.T. J. Littlewood and R. A. Bray, eds. Interrelationships of the Platyhelminthes. Taylor and Francis, London, United King- dom, p. 203–216.
Rohde, K. 1996. Robust phylogenies and adaptive radiations: A critical examination of methods used to identify key innovations. American Naturalist 148: 481–500. doi: 10.1086/285936
Swofford, D. L., G. J. Olsen, P. J. Waddell, and D. M. Hillis. 1996. Phylogenetic inference. In D. M. Hillis, C. Moritz, and B. K. Mable, eds. Molecular Systematics. Sinauer, Sunderland, Massachusetts, United States, p. 407–514.
Supplemental Reading
Baer, J. G. 1961. Classe des Temnocéphales. In P.-P. Grassé, ed. Traité de zoologie: Anatomie, systématique, biologie, Tome IV, Fascicule I: Plathelminthes, Mésozoaires, Acanthocéphales, Némertiens. Masson et Cie, Paris, France, p. 213–214.
Brooks, D. R. 1989. The phylogeny of the Cercomeria (Platyhel- minthes: Rhabdocoela) and general evolutionary principles. Journal of Parasitology 75: 606–616. doi: 10.2307/3282913
Jennings, J. B. 1971. Parasitism and commensalism in the Turbellaria. In B. Dawes, ed. Advances in Parasitology 9. Academic Press, New York, New York, United States, p. 1–32.
Tyler, S., ed. 1986. Advances in the Biology of Turbellarians and Related Platyhelminthes: Proceedings of the Fourth International Symposium on the Turbellaria (New Brunswick, Canada, August 5–10, 1984). [Reprinted from Hydrobiologia 132.]

