12 Chapter 12: Introduction to the Acanthocephala
Introduction
Members of the phylum Acanthocephala Rudolphi, 1808 are parasitic worms generally referred to as thorny-headed or spiny-headed worms because both larvae and adults have an invertible proboscis at the anterior end. However, this com- mon name is incorrect because acanthocephalans do not have heads! Although some consider the term head as only a gen- eral concept, it is not particularly useful in the area of invertebrate biology, except with those groups (such as Arthrop- oda) that actually have heads. For example, Maggenti and colleagues (2017) define a head as “The anterior body region.” which is not very useful to a biologist; however, in the same entry for head, 3 more definitions are included: Definition 2, referring to the polychaete annelids: The prostomium and the peristomium; definition 3, referring to the Arthropoda: Bearing the eyes, antennae, and mouth parts; definition 4, referring to the phylum Nemata: Comprising the lips and sensory organs, oral opening, and supporting head skeleton. Here, each definition is slightly more specific, focusing on the presence of particular structures and sensory organs as part of a head; these are more applicable to biology.
Why get so involved with definitions before acanthocephalans have even been described? There are several reasons, but only one very important reason is mentioned here. It is a complete theme in itself, namely, the concept of homology, meaning that 2 characteristics (structures, features, behaviors, and so on) are derived (evolved directly) from the same origin. Or, features, such as organs or structures in 2 or more taxa that can be traced back to the same feature in the common ancestor of these taxa. The concept of homology is in play every day when structures, characters, or features are called by the same name, indicating that they are the same thing, having similar features. Because what a name—such as head—means to us, we expect that it is similar to all other heads by having those important, recognizable features, such as having sensory organs clustered in that particular structure. Other obvious misnomers are the uterus, vagina, and penis of acanthocephalans, which are all names borrowed from vertebrate organs. Nevertheless, because they have been used since the first studies of acanthocephalans, scientists must use them or risk confusion.
To bring this back to animals that are known as belonging to the phylum Acanthocephala, the anterior ends of species in this group do not have a concentration of sensory organs—there are no eyes, no mouth, or any other elaborate sensory structures. Thus, to reiterate, the name spiny-headed worm is not appropriate because they have no heads! These conundrums of homology are problematic when trying to discover the relationships of this group to others, but is discussed as the phylogenetic relationships among the acanthocephalans, and the hypotheses about which groups might be close relatives, are considered.
Morphology
Compared to the bodies of members of many phyla of invertebrates, acanthocephalans are rather simple. However, the terminology relating to simple versus complex and primitive versus advanced are relative terms that are not often used by modern biologists for comparisons. This is because of the very nature of this comparison. For example, an acanthocephalan may be considered simple compared to a more complex annelid, but that same annelid is simple compared to most species of vertebrates. Thus, defining a species of organism as simple without context relative to the comparative morphological complexity of other species is futile.
With respect to simplicity versus complexity, this applies to acanthocephalans in relation to presence and absence of sensory structures and organs. First, consider what all spe- cies of the phylum Acanthocephala don’t have: First, there is no digestive system; second, there are no sensory struc- tures related to light detection and there are no sensilla (as in the phylum Nemata) for pressure detection that have yet been found. They have no organs or organ systems for the exchange of oxygen and carbon dioxide, and the majority of species investigated do not have protonephridia for excre- tion or water regulation. What they do have is discussed in the following sections.
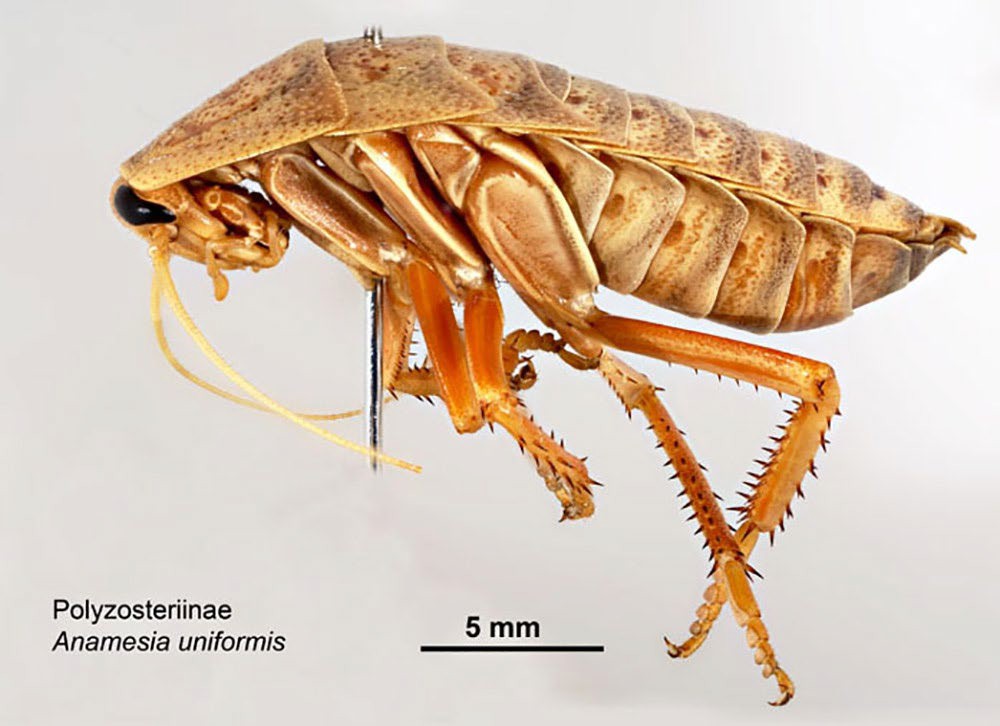
Figure 1. Anamesia uniformis (order Blattodea: family Blattidae: subfamily Polyzosteriinae) cockroach from Barrow Island, Western Australia. Cockroaches may serve as intermediate hosts for acanthocephalans. Source: L. Gibson and S. McCaffrey, Museums Victoria, Australia, 2006. License: CC BY-NC 4.0.
When it is said that acanthocephalans do not have elaborate sense organs, this does not mean that they cannot detect their environment. For instance, the larvae of many species are known to break out of their cysts in the stomach or anterior region of the small intestine of their vertebrate host, the region where bile empties into the intestine. However, this is not necessarily the site where they will establish themselves to begin to mate and produce eggs (Leadabrand and Nickol, 1993; Esch, 2000). One of the better-studied species is Leptorhynchoides thecatus, a parasite of the green sunfish Lepomis cyanellus. Detailed studies have shown that the young worms migrate through the intestine to the cecae of the fish (Richardson and Nickol, 1999; Richardson et al., 2008). They sense their surroundings, probably following chemical cues in the intestine and its contents and move anteriad and enter the cecae.
Hypotheses concerning how the Acanthocephala came to exist without these structures is discussed in a later section. Suffice it to say for now that the lack of common, or homologous, morphological structures or characters makes it difficult to estimate the phylogenetic relationship of species in this phylum with other groups of invertebrates.
Superficial External Features
Adult worms of most species of phylum Acanthocephala are fairly small—about 1 cm in length—but some individuals of many species are much smaller and individuals of some species may be really huge and can reach lengths of 70 cm (Miller and Dunagan, 1985a). Unstained by their surroundings, they are white, although some species can be colored yellow to or- ange by the carotenoids ingested by the intermediate (Figure1) or the definitive host (Nickol, 1985). In the host, the body of most species is somewhat flattened, but when the specimens are killed and fixed for study the osmotic pressure of this process fills the body cavity with liquid and it assumes a more cylindrical shape (Pritchard and Kruse, 1982).

Figure 2. Larval stage (cystacanth) of the fish parasite Pomphorhynchus tereticollis isolated from the paratenic host Neogobius melanostomus. A, C) Habitus of P. tereticollis, light- and scanning electron microscopy; B, D) detail of proboscis. Number and species specific structure details (arrows) of the proboscis hooks are clearly visible. Scale bar = 500 μm. Source: S. Emde et al., 2012. License: CC BY 4.0.
Cystacanths (the larval stage infective to the definitive host, specific to acanthocephalans; see Figure 2) are similar to adults except that the internal structures (reproductive organs and so on) are not fully developed. The cystacanths have developed into a form that is infective to the definitive host and then the development stops. Instead of being flattened, like adults of many species, the body of a cystacanth is more cylindrical in cross section. Mature cystacanths that are infective to the definitive host can be identified when the proboscis is completely inverted into the proboscis receptacle. The proboscis stays inverted until the definitive host ingests the cystacanth.
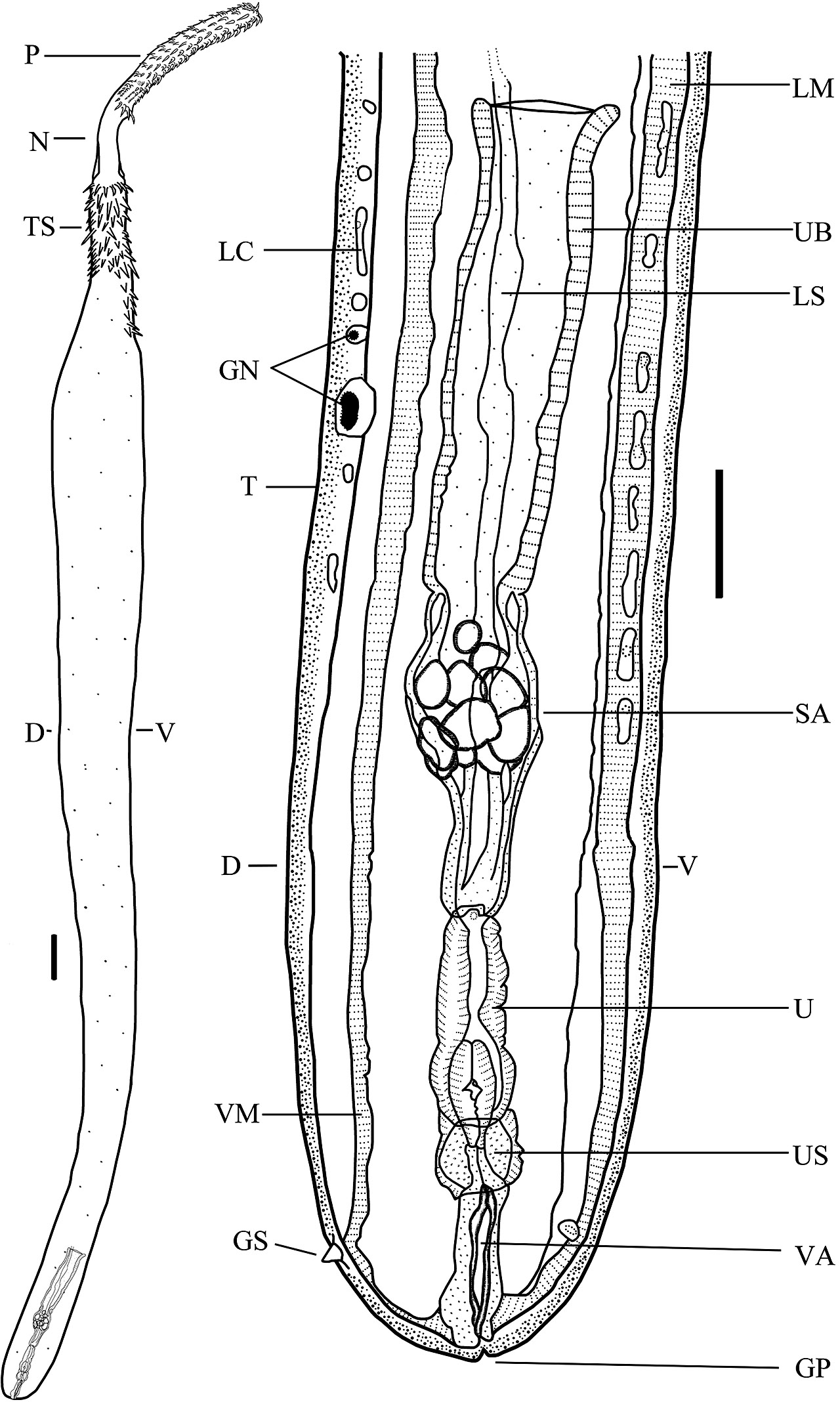
Figure 3. Drawing of the body and reproductive system of a typical female of class Palaeacanthocephala, Dollfusentis sp. D = Dorsal side of worm; GP = genital pore; GS = genital spine; LC = lacunar canal; LM = longitudinal muscles; LS = ligament sac; N = neck; P = proboscis with hooks; SA = sorting apparatus; T = tegument; TS = trunk spines; U = uterus; UB = uterine bell; US = uterine sphincter; V = ventral side of worm; VA = vagina; and VM = vestibular mus- cle. Source: S. Monks. License: CC BY-NC-SA 4.0.
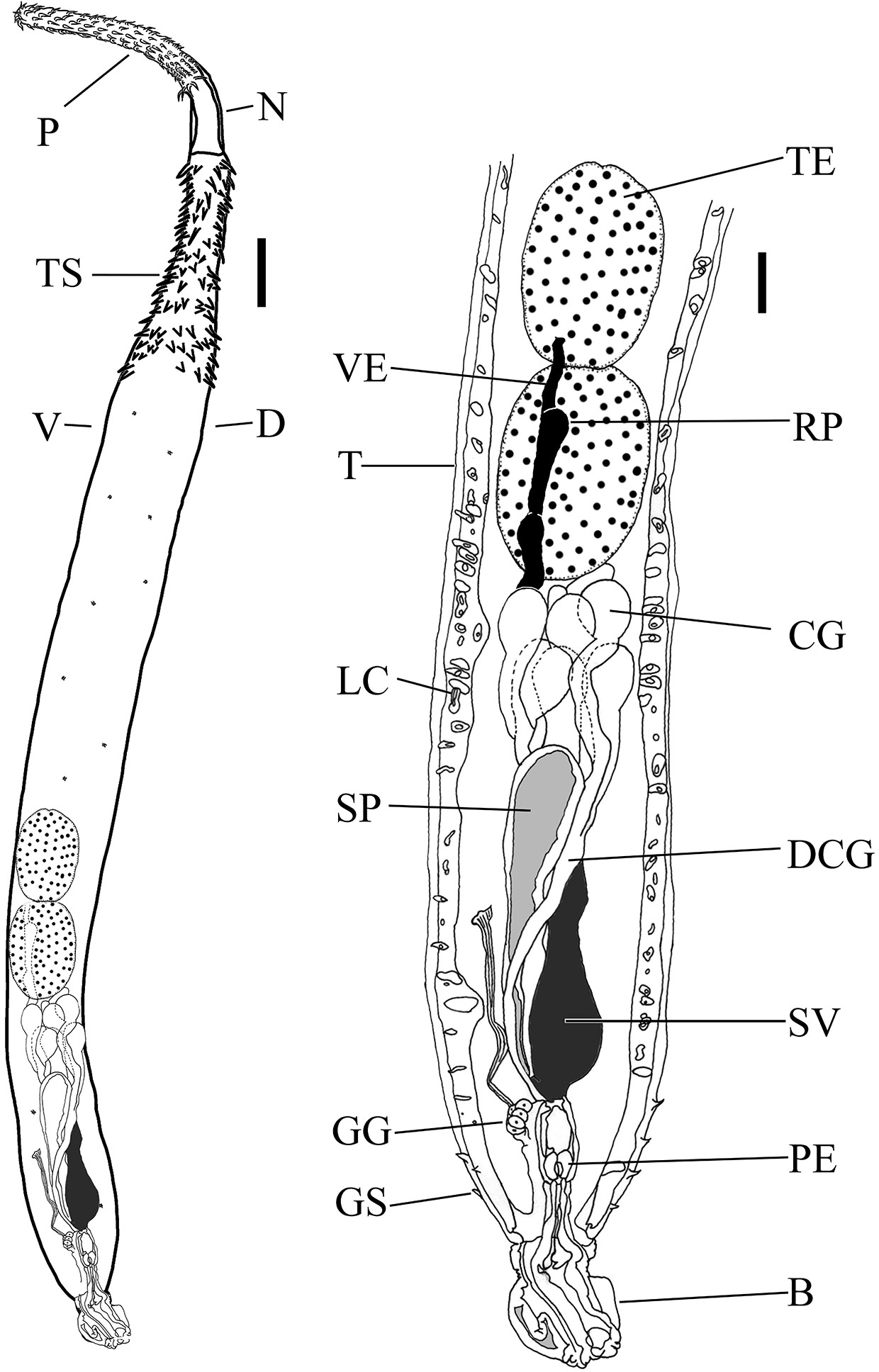
Figure 4. Drawing of the body and reproductive system of a typical male of class Palaeacanthocephala, Dollfusentis sp. B = Copulatory bursa (partially invaginated); CG = cement glands (8) (nuclei not visible); D = dorsal side of worm; DCG = ducts of cement glands; GG = genital ganglion; GS = genital spines; LC = lacunar canals; N = neck; P = proboscis; PE = penis; RP = pouch reservoir containing sperm; SP = Saeftigen’s pouch (gray color represents liquid in pouch); SV = seminal vesicle (black color represents sperm); T = tegument; TE = anterior testis; TS = trunk spines; V = ventral side of worm; and VE = vas eferens (black color represents sperm). Source: S. Monks. License: CC BY-NC-SA 4.0.
The outer surface of the body (called the trunk) of acanthocephalans can either be smooth or can have spines in the tegument. Spines are similar to hooks in composition but lack a root, which, in acanthocephalans, is an important tax- onomic character. The spines generally are in the more anterior part of the body, but in some species they also occur in the posterior part of the trunk in the area around the genital pore (Monks and Pérez-Ponce de León, 1996; Monks et al., 1997). The distribution of the spines can be continuous or in various patterns. These patterns are often characteristic for a species and can be used for identification (see the key in Amin et al., 2011).
General Structures of the Body
All acanthocephalans are quite similar in the general structure of the body (Figures 3 and 4). The body of all acantho- cephalans is composed of either 2 or 3 sections depending on whether the classic or modern designations are used.
In classic terminology, the body (also called the trunk; see Figure 5) is considered to be divided into 2 major regions, the praesoma and the metasoma. The praesoma comprises the armed (containing hooks) proboscis, proboscis receptacle, cerebral ganglion (Note: This should not be called a brain! Only vertebrates have brains), lemnisci, associated muscles, and the unarmed region between the proboscis and the rest of the body that is referred to as the neck (also, not a very good name for this region of the body). The metasoma is the hollow trunk of the body. The body wall, or tegument, of the metasoma encloses the body cavity.
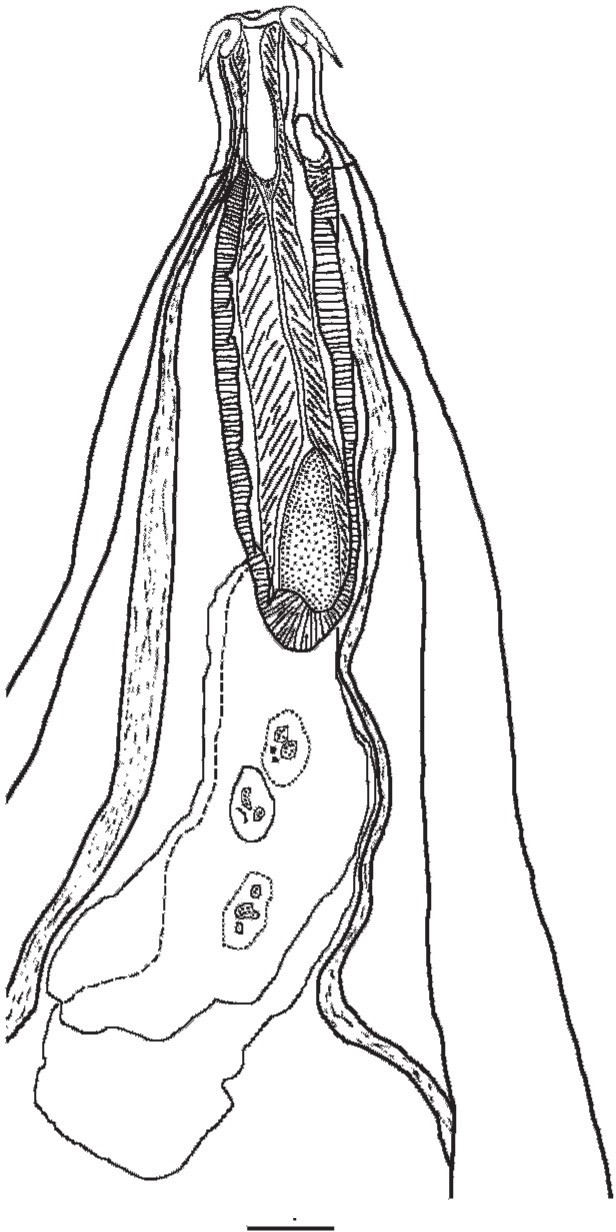
Figure 5. Anterior region of the body of a specimen of Neoechinorhynchus brentnickoli. Source: S. Monks. License: CC BY-NC-SA 4.0.
The tegument was previously called a pseudocoel (Miller and Dunagan, 1985a) but is now referred to as a persistent blastocoel, based on more recent studies of development (Brusca and Moore, 2016). A blastocoel is the hollow cavity that forms during embryonic development comprising a ball of cells. One part of this ball of cells then invaginates, forming a mouth or an anus (depending on which group of animals are being discussed); this is called gastrulation and the larval form is called a gastrula. The body cavity of an acanthocephalan is the remnant of this hollow ball of cells that did not completely become lined with mesodermally-derived tissues during embryogenesis.
The tubular channels that constitute the lacunar system infiltrate the entire tegument of the metasoma and 2 major canals extend from the anterior to the posterior end of the trunk.
Species included in some groups have spines that are distributed over the trunk in various patterns. These spines are similar to hooks but are smaller and do not have roots. Within the body cavity are the proboscis receptacle, the associated muscle bands mentioned above, and the reproductive organs. Acanthocephalans are gonochoristic. Associated with the reproductive organs of males are cement glands, Safftigen’s pouch, an evertible/retractable bursa at the posterior end, associated ducts, among other structures. Females lack these structures. Each group of structures and organs is discussed separately.
Tegument is living tissue that is a syncytium of cells without nuclei which includes dense fibers and connective tissue. The body of acanthocephalans is covered by a multilayered tegument, the overall structure of which resembles that of rotifers (Herlyn et al., 2003; Weber et al., 2013; Sielaff et al., 2016; but see Dunagan and Miller, 1991 for a traditional interpretation). Underneath the tegument are circular and longitudinal muscles, many of which are tubular rather than a dense solid mass. The outer surface of the tegument contains numerous micropores that connect to fine canals leading to a complex system of tubes that extend throughout the tegument in patterns specific to particular groups of acanthocephalans. As mentioned above, the system of tubules is called the lacunar system. Finally, the tegument may contain nuclei, called giant nuclei in some taxa. There can be a few very large/giant nuclei or more numerous branched nuclei (for examples of giant nuclei see figures in Monks et al., 2011, and branched nuclei in those of Monks et al., 1997).
Morphology of the Praesoma
The proboscis is one of the distinctive structures of acanthocephalans. The armament (hooks and spines; Figure 6) is a distinctive feature of the proboscis. It can be withdrawn into the proboscis receptacle (within the body cavity) by turning it inside out. The hooks make the invagination process necessary.
The hooks somewhat resemble the thorns on the stem of a rose. In most species the hooks are curved, although in some, the more posterior hooks may extend almost perpendicular to the proboscis rather than be curved posteriad (see the fig- ures in Amin et al., 2011). Some species, such as Koronacantha mexicana, have rootless spines posterior to the hooks (see the figures in Monks et al., 1997). Each hook consists of a root (the part which anchors the hook to the proboscis) and the blade (the pointed part of the hook). The root is only an anchor for the hook, not to be confused with the root of a plant. That is, there are no other structures or muscles that might enable the movement of the hook. Spines on the proboscis do not have roots; those on the trunk also don’t have roots. This distinction, that hooks have roots and spines do not have roots, seems clear, but in real life the difference often is blurred. Finally, some prefer to call the spines on the proboscis rootless hooks (Muñoz and George-Nascimento, 2002), leaving the term spines for the armament on the trunk. When the hooks are fastened into the intestinal wall of the definitive host of the worm, there is no way to dislodge the hooks. This is why the process of invagination of the proboscis is necessary. To visualize the retraction of the proboscis back into the body cavity of the acanthocephalan, if one imagines that a hand is inserted into a tight-fitting glove, it is obvious that the hand is not easily withdrawn from the glove. The easiest way to remove the glove is to turn the glove inside out, removing it by pulling the part nearest the wrist distally toward the fingers and finally over the fingers and off the hand, leaving the glove inside out. The folding inside out of the glove is similar to what happens to the proboscis in invagination.
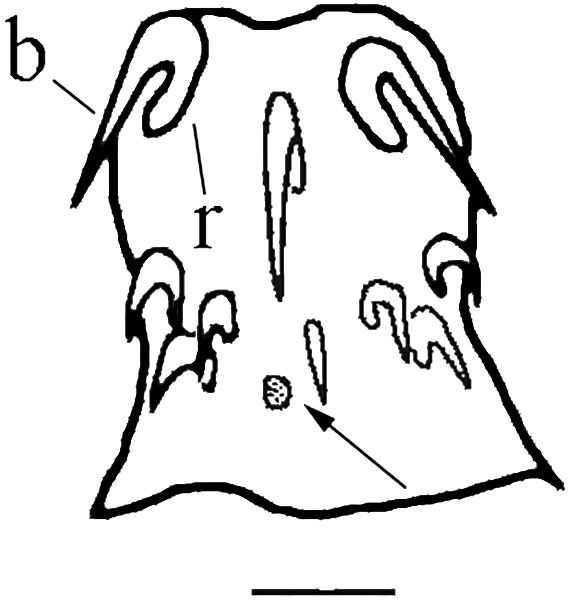
Figure 6. Proboscis and neck of a specimen of Neoechinorhynchus brentnickoli. b = blade of hook; r = root of hook; arrow indicates lateral sensory pore. Scale bar = 25 µm. Source: S. Monks. License: CC BY-NC-SA 4.0.
In the definitive host, the worm forces its proboscis into the tissue of the wall of the host’s intestine. Once inserted, the hooks prevent removal and protect the worm from being dislodged by movements of the intestine or its contents. However, acanthocephalans move and migrate within the host’s intestine (Leadabrand and Nickol, 1993; Richardson et al., 2008) so they need to be able to unhook themselves. Long strands of inverter muscles extend from inside the body cavity anteriorly to the most anterior point of the proboscis. Normally they are relaxed, permitting the proboscis to remain inserted firmly into the wall of the host’s intestine. When an individual acanthocephalan prepares to move, the inverter muscles contract and pull the proboscis inside of the receptacle, disconnecting each ring of hooks as the proboscis is invaginated. This smoothly removes the hooks opposite the way they went in rather than by forcibly tearing them out.
The proboscis of acanthocephalans has different shapes in the different members of the phylum. In general, the pro- boscis is cylindrical or spherical. In a phylogenetic analysis of members of the phylum, Monks (2001) identified different shapes of the proboscis of the species included in his analyses, including: Round, elliptical to oval, elongate to fusiform, clavate, and cone-like. These shapes were sufficient to differentiate between the taxa in those analyses. Although other shapes are known, such as, spindle-shaped, meaning, wide in the middle and tapering toward each end (see the figures in Richardson et al., 2010, for an example) that would be needed if more species had been included in the analyses by Monks (2001).
The function of the proboscis is to provide attachment to the intestinal wall of the definitive host by penetrating the in- testinal wall. To date there is no hypothesis that relates the qualities of the intestinal wall to a particular shape of probos- cis. One might think that the length and shape of the proboscis would be related to the structure of the host’s intestinal wall—that is, thickness, muscularity, presence of thick con- nective tissue, and so on—but this does not seem to be true. For example, the proboscis of Macracanthorhyncus hirudinaceous, which is a parasite of pigs, has a relatively small and round proboscis (similar to that of Neoechinorhynchus brentnickoli). In contrast, species of Pomphorhynchus, which are parasites of fish, have a medium-sized, cylindrical proboscis and a long neck that penetrates the relatively thin intestinal wall and extends into the body cavity (see photos and discussion at https://alchetron.com/Pomphorhynchus-laevis). Other than the hooks, the extent of which marks the posterior margin of the proboscis, few other structures are included in the proboscis (Miller and Dunagan, 1985a). Internally, at the anterior end of the proboscis is a small group of cells called the apical organ (Miller and Dunagan, 1983; 1984). The apical organ varies in shape depending on the group, and in some there is a small pore leading outside of the proboscis (Dunagan and Miller, 1983). Note that the apical organ discussed here is not homologous with the apical organ that is found in all species of tapeworms of the genus Hymenolepis. In all species of phylum Acanthocephala that have been examined there are 2 large nuclei in the posterior area of the apical organ.
Located opposite each other on the lateral sides of the proboscis, usually near the posterior-most ring of hooks, is a pair of sensory pores (Figure 6), or lateral sense organs (Herlyn et al., 2001), that open on the surface of the proboscis. Internally, the pores are connected to the sensory support cell complex (Miller and Dunagan, 1983; 1984; 1985a). The func- tion of these cells is not well understood and it is not known what they detect.
Neck
The neck is relatively featureless and there are no hooks or spines present. The posterior margin of the proboscis in- cludes the posterior-most hooks or spines, and thus is not the neck. The neck is tubular, hollow, and connects the proboscis to the trunk. Structures (muscles, nerves, and in some cases the proboscis receptacle) pass through the hollow center of the neck, but they are not fastened to it. Species of Pomphorhynchus, which are parasites of adult fish, are one excep- tion (see the photographs and life cycle diagrams available at https://alchetron.com/Pomphorhynchus-laevis). These species have the neck enlarged to form a bulb. The proboscis penetrates the intestinal wall of the host fish, often extending into the body cavity, and the bulb expands to prevent the proboscis from being dislodged.
Morphology of the trunk (metasoma)
The proboscis may be the most notable structure of acanthocephalans, but the trunk, which constitutes the rest of the body, contains the majority of structures. The trunk is divided from the neck by the attachment of the proboscis receptacle.
The lacunar system, which is the canal system of the trunk, starts anteriorly at the neck-trunk junction and extends to the posterior end of the body. Longitudinal canals run dorsally and ventrally, or laterally to link circular canals (Miller and Dunagan, 1985a; 1985b). It is thought that the liquid in the canals circulates as a result of body movements.
As mentioned above, many species have spines on the surface of the trunk, distributed in various patterns. Only recently, studies of the manner of attachment to the intestinal wall by species of Corynosoma have shown that the spines assist in providing a secondary attachment (Aznar et al., 2002; 2016).
The proboscis receptacle is attached to the anterior portion of the trunk. The receptacle, as the name implies, is a structure in which the proboscis is retracted into when it is inverted, but this seems to be only a secondary function because the proboscis could just as well be drawn into the body cavity. The receptacle is a sac, open at the anterior end and most commonly attached at the neck-trunk junction. However, in some taxa the receptacle is attached at the posterior ring of proboscis hooks (see the figures in Amin et al., 2017), or, in a few groups, in the middle of the proboscis (see the figures in Richardson et al., 2010). The wall of the receptacle is composed of 1 or 2 layers of muscle. The muscles of each layer have fibers that are circularly, longitudinally, or spirally oriented (Monks, 2001). Long retractor muscle bands attach to the anterior end of the proboscis and they extend posteriad through the posterior end of the receptacle and are attached to the inner surface of the body wall. When these bands contract, they pull the proboscis into the receptacle. There are no antagonistic muscles that can pull the proboscis back out. Eversion of the proboscis is accomplished by contraction of the muscular receptacle walls, evidently forcing the proboscis out by hydrostatic pressure. Several other muscle bands pass through the receptacle, but most importantly, the cerebral ganglion is found in the receptacle. The cerebral ganglion hangs from nerves that run anteriad from it. These nerves exit the receptacle and attach to the inner wall of the trunk, running posteriad. The ganglion is composed of a small number of neurons (around 100 of them), although this knowledge is based on studies of just a few species (Miller and Dunagan, 1985a).
The paired lemnisci are connected anteriorly at the neck/ trunk junction. Each is a long, spongy organ with a few giant nuclei. The function of the lemnisci is unknown, leading some investigators to associate them with the lost digestive system, and possibly with the salivary glands of rotifers (Miller and Dunagan, 1985a). Moore (1946) observed that the lemnisci develop as evaginations of the hypodermal layer of the trunk in Moniliformis moniliformis. To date, the best interpretation of the function of the lemnisci is derived from observations of the lemniscal plasmalemma membrane that has numerous infoldings that greatly amplify the free surface area exposed to the metasomal (body) cavity (Wright, 1970). Thus, it is thought to have an important physiological role in transporting material relative to the metasomal cavity. However, the precise nature of the materials being transported has not been investigated.
Morphology of the reproductive organs
As in all parasitic worms, the reproductive organs are well-developed structures and are obvious in stained, cleared, and mounted specimens. In acanthocephalans, this is especially true because in the majority of species, the trunk cav- ity is almost empty except for the presence of the reproductive system. As mentioned above, these animals are dioecious or gonochoristic, meaning that the sexes are separate, with male and female individuals.
Associated with the reproductive system are the genital ganglia and the protonephridia. The genital ganglion is a small nexus of neurons that is presumed to control the male reproductive organs (Dunagan and Miller, 1978; Dunagan and Price, 1985); however, in most descriptions of species, the genital ganglia are not mentioned. Most species of acan- thocephalans do not have protonephridia, but in a few species, females possess protonephridia comprising flame cells (Miller and Dunagan, 1985a).
Female Reproductive Organs
The main reproductive organs of females are, from anterior to posterior: Ovary, uterine bell, sorting appara- tus, uterus, vagina, and gonopore. The ligament sac is associated with the reproductive system and is a hollow, membranous tube—in some groups there are 2 sacs—that runs from the proboscis receptacle to the uterine bell. As the worms mature, the sacs persist in species classified in the class Archiacanthocephala but they rupture in species of both classes Palaeacanthocephala and Eoacanthoceph- ala. In some species almost no remnants can be found. The ovaries develop within the ligament sac. The evolutionary origin of the ligament sac is uncertain, but the presence of the ovaries within the lumen of the sac precludes identifying them as the missing intestine.
When female acanthocephalans are immature, they first have 1 ovary that fragments into groups of cells called ovarian balls, which subsequently continue fragmentation into ova (unfertilized eggs) and finally, when fertilized in mature females, into shelled eggs, which are also called shelled acanthors because the embryo is called an acanthor. The unfragmented ovary can only be seen in female cystacanths or very immature adults. Asaolu (1980) and Asaolu and colleagues (1981) completed detailed studies of this process, including scanning electron micrographs.
While developing, fertilized ova circulate within the unbroken ligament sac or within the body of those species in which they do not persist. Eventually, eggs with shells, both immature and mature, enter the uterine bell. Note that the eggs are mature when they are infective to the intermediate host and only mature eggs are passed into the intestine and then out into the external environment; this is the function of the sorting apparatus. The chemical or physical indicators of maturity are unknown, but the apparatus has 2 openings, one leading back to the body cavity and one leading to the uterus. Based on whatever clues are used, the sorting apparatus sorts the eggs, with the immature ones being routed back to the body cavity for further development and the mature ones being sent on to the uterus.
Mature eggs in the uterus pass to the vagina and then, one by one, out to the environment (which comprises the fecal material in the intestine of the definitive host). Although not a part of the reproductive system, all females have muscles located near or around the gonopore (see Monks and Pérez-Ponce de León (1996) for drawings of the vestibular muscle of Koronacantha mexicana). Monks (2001) identified 10 different types of muscle of what has been called the genital vestibule. The acanthocephalans have not been studied sufficiently to identify patterns of the evolution of the different forms of vestibular muscles, but these structures may be important in protecting females of one species from being inseminated by males of a different species. Despite the many different forms, all appear to have the same function—to change the shape of the region around the genital pore in order to prevent copulation until the female is ready or not to permit the bursa of males to fit over the posterior end of females, which, in turn, prevents the penis of males from connecting to the genital pore (see figures in Monks et al., 2008).
Male Reproductive Organs
The principle reproductive organs of males are (from anterior to posterior): Testes, sperm ducts, sperm reservoir, and penis. Associated structures are: Cement glands, cement reservoirs (if present), Saeftigen’s pouch (also spelled Seftigen), genital ganglia, and bursa. A thorough study of the morphology of the reproductive system of males is provided by Asaolu (1981).
Male acanthocephalans have 2 testes, variable in location but always located some distance anterior to the remaining organs. As noted by Monks (2001), they are located generally in tandem with one another but they can be almost in line or more diagonal, distant from each other, or somewhat overlapping, but they are never opposite one another. Each testis is connected by the vas efferens to either the vas deferens or directly to the seminal vesicle, depending upon which group they belong to. A duct connects the seminal vesicle to the penis. The vasa efferentia may be expanded in some region to provide additional storage for sperm. Occasionally, males may only possess a single testis, a monorchia, although it is not common. Miller and Dunagan (1985a) provide a list of reports of monorchidism in various species.
The male members of many invertebrate phyla, and some females of those phyla, possess cement glands. Typically, the cement is used to bond an organism to a substrate, anchoring the organism so it is not dislodged. The cement from the glands of male acanthocephalans also is used for anchoring, but not to a substrate; instead, it is used to glue them tempo- rarily to a female during copulation. The cement also serves to close the gonopore of females, although it is only tem- porary and it subsequently deteriorates, allowing females to mate again at a later time.
Several types of cement glands are known: A single syncytial gland, usually with 8 giant nuclei; a small number (usually 2–8) of glands, each with a single giant nucleus; or a small number of glands that have numerous fragments of nu- clei in each (Van Cleave, 1949).
Contrasting views of the evolution of the cement glands have been suggested, but modern phylogenetic analyses indicate that separate glands with single nuclei are plesiomorphic and a syncytial gland has been shown to be a synapomorphy for the species included in the class Eoacanthocephala (see Monks, 2001).
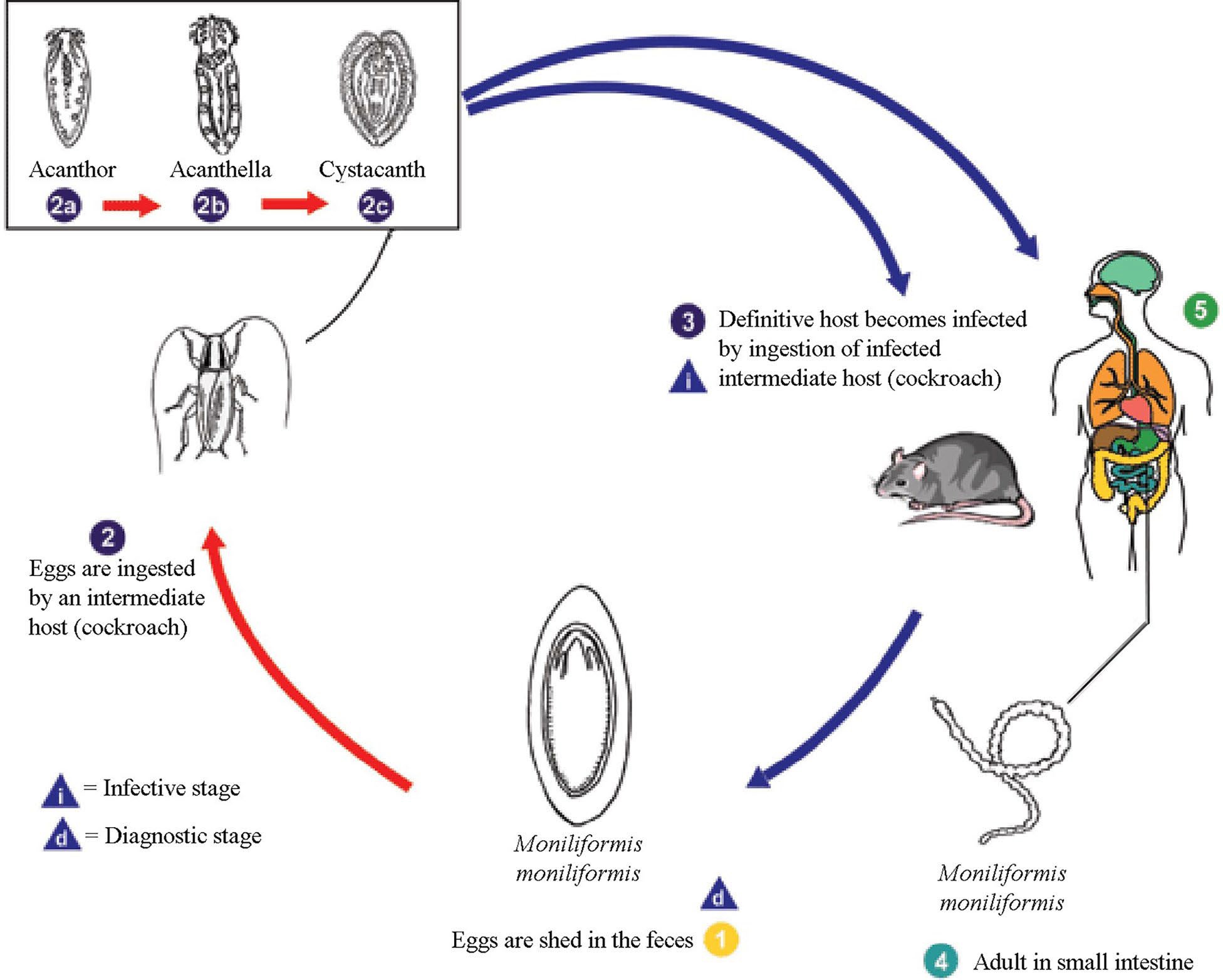
Figure 7. Life cycle of Moniliformis moniliformis. 1) Eggs are shed in the feces of the definitive hosts, which are usually rats for M. moniliformis, although carnivores and primates, including humans, may serve as accidental hosts. The eggs contain a fully-developed acanthor when shed in feces. 2) The eggs are ingested by an intermediate host, which is an insect (cockroaches, Periplaneta americana, for M. moniliformis). Within the haemocoel (persistent blastocoel) of the insect, the acanthor (2a) molts into a second larval stage, called an acanthella (2b). After 6–12 weeks, the worm reaches the infective stage, called a cystacanth. The definitive host becomes infected upon ingestion of intermediate hosts containing infective cystacanths (2c). Note that the proboscis is inverted. 3) The definitive host becomes infected by consuming an infected intermediate host. In the definitive host, larvae are liberated from their cysts and they attach to the wall of the small intestine. 4) Here they mature and mate in about 8–12 weeks. 5) In humans, the worms seldom mature, or, when they do mature, will rarely produce eggs. Source: Adapted from Division of Parasitic Diseases and Malaria, United States Centers for Disease Control and Prevention, 2019. Public domain.
Saeftigen’s pouch is an expandable vesicle that is con- nected to the bursa. The bursa normally is inverted within the posterior body cavity of males. The muscular Saeftigen’s pouch contains liquid that is pumped into the bursa, forcing the bursa out into the form of a cuplike structure that covers the posterior of females and aligns the penis with the vagina. Cement is then released into this area to seal the 2 individuals in copula. Although the exact modes of neural communications are not known, the genital ganglion probably controls the performance and sequences of the various genital organs. However, it is interesting that a similar organ has not been reported in females.
Life Cycles
Compared to other groups of helminths (that is, subclass Digenea, class Cestoda, phylum Nemata, and others) a typical life cycle of acanthocephalans is relatively simple to learn— the definitive host is always a vertebrate and the intermediate host is always an arthropod. A typical life cycle, that of Moniliformis moniliformis, is shown in Figure 7. Acanthocephalan life cycles are linked to trophic relationships. This means that a definitive host becomes infected by ingesting its normal food that has a larval acanthocephalan that is infective to that particular species of definitive host.
For this reason, many species of Acanthocephala have very narrow host ranges. Using the previous example, a bird that feeds on insects that eats a cockroach infected with Moniliformis moniliformis will not become infected. Likewise, neither will a rodent feeding on pillbugs Armadillidium vulgare infected with cystacanths of Plagiorhynchus cylindraceus, which normally occurs in the robin Turdus migratorius (see Coady and Nickol, 2000 for a study of this type of interaction). However, this elucidates one curiosity of acanthocephalans. In this latter case, upon ingestion, the helminths would migrate out of the intestine into the body cavity of the rodent. While there, the cystacanth does not develop further; however, it may re-encyst in the rodent where it remains, in a kind of stasis, until a proper host comes along that it can infect, which in this case is probably never, unless of course the rodent dies and an isopod feeds on the dead rodent then becoming in- fected, ready to transfer the infection on to the avian final host. As mentioned above, all parasite life cycles are trophically linked and in the cases discussed here, only arthropods can function as intermediate hosts. This would preclude any spe- cies that does not eat arthropods from being infected with acanthocephalans. However, there are cases in which the defin- itive host (such as a hawk or an owl) does not eat arthropods but those species can become infected naturally—here enters the paratenic host. The paratenic host is an ecological bridge between the arthropod intermediate host and the definitive host that does not eat arthropods. Usual paratenic hosts are small, insect-eating vertebrates, or in the case of fish, small fish that eat very small aquatic crustaceans; that is, frogs, toads, small lizards, snakes, rodents, and other small fish.
One would never think of a noble eagle or hawk eating insects, but they still can be infected with acanthocephalans. An example of a life cycle of species that involves paratenic hosts is that of the owl dwelling acanthocephalan called Cen- trorhynchus, of which there are several species. Insects become infected when they ingest eggs in the feces of the definitive host (owl or hawk). Snakes, frogs, and/or toads eat the insects, and a lot of them. The cystacanths in the infected insects excyst in the intestinal lumen and migrate to the body cavity where, as mentioned above, they re-encyst. They stay there, alive but in a type of hibernation, until a predatory bird captures the paratenic host, whereupon the cystacanths excyst again and develop within the bird. As an example of the complexity of the situation, Tavares dos Santos and Amato (2010) studied a life cycle in Brazil involving a species of Centrorhynchus and a toad, Rhinella fernandezae. The definitive host has not yet been identified, but several species of Centrorhynchus occur in Brazilian birds.
Finally, it is important to note again that there is no development of cystacanth larvae in paratenic hosts. If the definitive host, such as an eagle or the fish mentioned above, was fed an infected insect or crustacean, respectively, it would become infected with the acanthocephalan, just as it does when it eats the paratenic host.
Before leaving life cycles, one might wonder why there is relatively little precise information on more acanthoceph- alan life cycles. To give an example, when J. R. Crook was a graduate student, he captured specimens of Peromyscus ma- niculatus (commonly called a deer mouse) and found them to be infected with adult acanthocephalans, Moniliformis clarki. Imagine the difficulty in figuring out what arthropods the mouse might be eating, particularly because it is an omnivore. Eventually, Crook discovered that the mice were catching and eating crickets, Ceuthophilus utahensis, the Utah camel cricket, that lived in the underground tunnels that the mice made in which to live. The mice set aside a space in the tunnel where they defecated, and the crickets would go there and eat the feces, some of which carried eggs of Moniliformis that were passed in the feces of the mouse. The mice would then catch and eat infected crickets, completing the life cycle. This is not the most obvious place to look to find insects on which the mouse was feeding, unless the general life cycle of acanthocephalans was known and if the natural history of the mouse itself was known (Crook and Grundmann, 1964). Searching out the participants in a life cycle is difficult and often might be the result of luck! This points out a second problem. The parasitologist, who might be a specialist in helminths, must also be a specialist in the vertebrate species that are definitive hosts for the helminths they study, and must know where they live and what they eat. In the case of acanthocephalans, the parasitologist must also know the arthropods, where to find them, and how to identify them. Much of this is not obvious when one reads the description of a life cycle. Today, molecular techniques often are used to match up the identity of cystacanths with adult worms, which often cannot be identified using only morphological details of the cystacanth larvae. Such a study was carried out by Lorenti and colleagues (2018).
Classification and Phylogenetic Relationships
The classification of the members of the phylum Acanthocephala has been relatively stable for some time, but understanding of the phylogenetic relationships of acanthocephalans and relationship to other invertebrate taxa has been in flux significantly. This is largely because the classification of the phylum is still grounded upon classical inductive interpretation of how acanthocephalans should be grouped based on particular characteristics. A list of classical characteristics is presented in Table 1 (Bullock, 1969). These and, of course, other characters have been thought to be indicators of similar ancestry. Thus, species with these characters were placed in the same group (classes are indicated in the table).
A phylogenetic hypothesis, on the other hand, is the result of an analysis of data without the a priori decisions that classical reasoning might give (even though the two might be consistent). It is a provisional conjecture to guide further investigation, although it can be accepted as highly probable based on sound analyses, in view of established facts (data). Instead of using similarity, the hypothesis of relationships is based on characters that are homologous. However, the same classical data might be used in a phylogenetic analysis but the methodology is completely different (see Monks, 2001, for a partial list of the type of data that are useful for this type of analysis).
Because of the different methodology, classifications are rarely 100% consistent with phylogeny, although it would be advantageous if they were consistent. However, thanks to the intellectual acuity of the classical experts who studied acanthocephalans, the classification and recent phylogenetic hypotheses for the higher taxa are relatively similar. Several works provide complete classifications of the phylum Acanthocephala (Amin, 1985; 2013; Golvan, 1994).
Each recognizes the 3 classical classes, Archiacanthocephala, Eoacanthocephala, and Palaeacanthocephala, and some add a fourth class, Polyacanthocephala (though others view it as a part of Palaeacanthocephala). Interestingly, the 3 names are tied to early views that one or the other was the most ancient taxon. For those interested in classical classification of the phylum it would be worthwhile to consult the works of Petrochenko (1956; 1958) and Yamaguti (1963). The most recent compendium discussing all aspects of acanthocephalan biology, including classification, is Crompton and Nickol (1985). For a list of higher taxa and the number of species known from each at the time of publication, see Monks and Richardson (2011).
Phylogenetic hypotheses of the Acanthocephala are largely consistent with the arrangement of higher taxa, with a continuing greater resolution of relationships and changes in placement as studies have advanced. The first phylogenetic hypothesis for a partial group of genera representing the 3 classes using molecular data were Near and colleagues (1998) and García-Varela and colleagues (2000). The first hypothesis based on molecular data was Monks (2001). Despite some differences in the inclusion of taxa and the methodology, the results of the 3 are similar. In each, Eoacanthocephala and Palaeacanthocephala are designated to be monophyletic sister taxa, meaning, 2 taxa that descended from the same most recent common ancestor. Archiacanthocephala is the most basal class in both cladograms, but in one (Figure 8A) it is a monophyletic clade and the members do not form a monophyletic group (Figure 8B) (see the cladograms in García-Varela et al. (2000), Near et al. (1998), and Monks (2001), respectively).
Table 1. Characterization of the 3 orders in the phylum Acanthocephala. Adapted from Bullock, 1969.
|
Character |
Archiacanthocephala |
Eoacanthocephala |
Palaeacanthocephala |
|
Body size |
Mostly large |
Small |
Small to large |
|
Host habitat |
Terrestrial |
Aquatic |
Mostly aquatic |
|
Lacunar system, |
Dorsal and ventral or dorsal only |
Dorsal and ventral, at least anteriorly |
Generally lateral |
|
Cement glands |
Usually (always?) 8 uninucleate |
Usually 1, syncytial, with giant nuclei; distinct cement reservoir |
From 2 to 8, multinucleate |
|
Trunk spines |
Absent |
Present or absent |
Present or absent |
|
Subcuticular nuclei |
Few, elongate or branched, or with fragments remaining close together |
Very few giant nuclei |
Numerous amniotic fragments or few highly branched |
|
Proboscis receptacle |
Single muscle layer, often modified by ventral cleft or accessory muscles |
Closed sac with single muscle layer |
Closed sac with 2 muscle layers, except in subfamily Polyacanthorhynchinae |
|
Ligament sac |
Dorsal and ventral, persistent, with dorsal sac attached to uterine bell |
Dorsal and ventral; disappear in adult; ventral sac attached to uterine bell |
Single, ruptured in mature worms; posterior attachment inside uterine bell |
|
Nephridia |
Present or absent |
Absent |
Absent |
|
Embryonic membrane |
Usually thick |
Thin |
Usually thin |
|
Intermediate host |
Insects (and millipedes) |
Subphylum Crustacea |
Subphylum Crustacea |
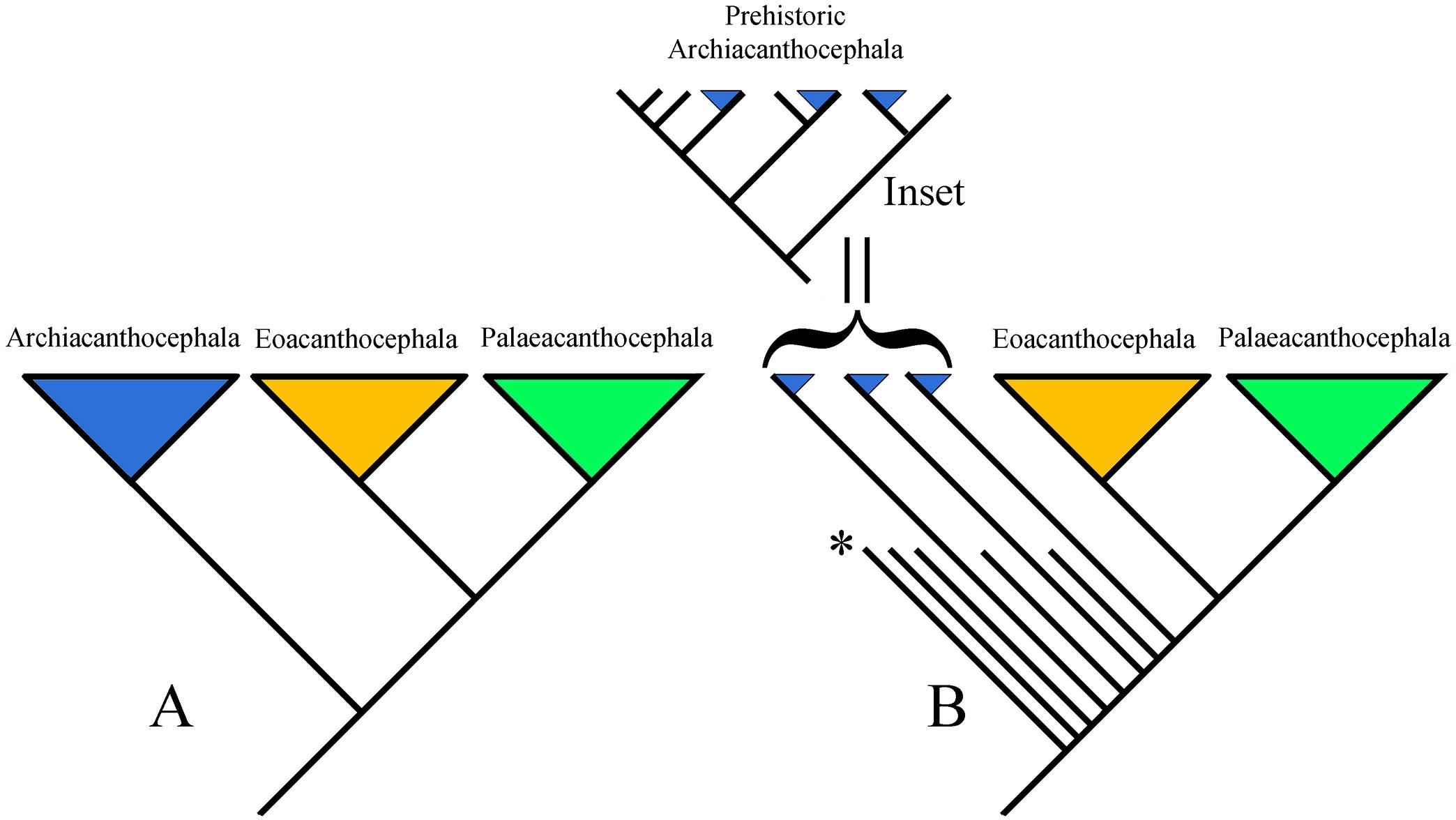
Figure 8. Hypotheses of the phylogenetic relationships of the phylum Acanthocephala. A) Cladistic representation of the general results of 2 molecular analyses; B) Cladistic representation of the general results of another morphological analysis. Inset: Hypothetical clade of prehistoric archiacanthocephalans that in nature represented a monophyletic clade. The asterisk (*) indicates extinct taxa, represented by shorter lines. In the inset, the lines without triangles represent extinct taxa. The clade shown is a hypothetical monophyletic clade including extinct taxa. In this clade none of the extant taxa are closest relatives; the sister groups of each are extinct. Synapomorphies for this putative clade have not been identified; thus, the 3 branches with blue triangles (extant archiacanthocephalans) cannot be identified as a monophyletic clade. Sources: A) Adapted from García-Varela et al., 2000; Near et al., 1998; B) adapted from Monks, 2001. License: CC BY-NC-SA 4.0.
The failure of the methodology to recognize the class Archiacanthocephala as a monophyletic group was interpreted by Monks (2001) as an artifact caused by the very old origin of acanthocephalans. Monks suggested that present day taxa (Figure 8B) are only a relict of the original species in the group (Brooks and Bandoni, 1988); that is, many of the original species (and their hosts) are extinct (Figure 8B, in- set) and their absence from the analysis hindered the ability of the methodology to identify synapomorphies for the clade. Interestingly there are studies that have been interpreted that indicate that acanthocephalans are a part of the phylum Rotifera. One of the first was by Herlyn and colleagues (2003). To continue to explore this interpretation, refer to that study and the subsequent works, both pro and con, which cite this study. Earlier studies (Conway Morris and Crompton, 1982) postulated the phylum Priapulida as a sister group to Acanthocephala, but this idea mainly was based on similarity of fossil priapulids with present-day acanthocephalans.
This summary is far from providing a complete picture of this fascinating group. For more information there are nu- merous published papers available on the internet or in university libraries, only a very few of which were cited here. Many of these are descriptive taxonomic works, but there are also studies on physiology, behavior, ecology, and more on the subjects mentioned above.
The information and interpretations presented here are based on a phylogenetic perspective. For more information on phylogenetics, terminology, and methodology, a great source is The Compleat Cladist (Wiley et al., 1991; available as a free PDF download at https://kuscholarworks.ku.edu/han- dle/1808/24957). For further information on phylogenetic hypotheses of different groups of helminth parasites, see Brooks and McLennan (1993; 2002). For sources which bring ecology, behavior, biogeography, and other areas of biology together in a phylogenetic perspective, see Brooks and McLennan (1991). Searching in the Web of Science or Google Scholar for sources that cite these works will provide more recent sources of information.
Original Textbook Chapter 58 Authors
Scott Monks
Universidad Autónoma del Estado de Hidalgo, Centro de Investigaciones Biológicas, Pachuca, Hidalgo, Mexico; and Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States scottmonks@hotmail.com
Reviewer: Michael A. Barger, Department of Biology, Health Science, and Integrative Human Biology, School of Health Sciences, Stephens College, Columbia, Missouri, United States
Revisions: Reordered for lecture and lab.
Literature Cited
Amin, O. M. 1985. Classification. In D. W. Crompton and B. B. Nickol, eds. Biology of the Acanthocephala. Cambridge University Press, Cambridge, United Kingdom, p. 27–72.
Amin, O. M. 2013. Classification of the Acanthocephala. Folia Parasitologica 60: 273–305. doi: 10.14411/fp.2013.031
Amin, O. M., R. A. Heckmann, and P. A. A. Shareef. 2017.Redescription of Pallisentis (Brevitritospinus) indica (Acanthocephala: Quadrigyridae) from Channa punctatus Bloch & Schneider (Channidae) in Aligarh, India with new understandings of old structures. Journal of Parasitology 103: 251–256. doi: 10.1645/16-153
Amin, O. M., R. A. Heckmann, and N. Van Ha. 2011. Description of two new species of Rhadinorhynchus (Acanthocephala: Rhadinorhynchidae) from marine fish in Halong Bay, Vietnam, with a key to species. Acta Parasitologica 56: 67–77. doi: 10.2478/s11686-011-0004-3
Asaolu, S. O. 1980. Morphology of the reproductive system of female Moniliformis dubius (Acanthocephala). Parasitology 81: 433–446. doi: 10.1017/S0031182000056158
Asaolu, S. O. 1981. Morphology of the reproductive system of male Moniliformis dubius (Acanthocephala). Parasitology 82: 297–309. doi: 10.1017/S0031182000057048
Asaolu, S. O., P. J. Whitfield, D. W. T. Crompton, and L. Maxwell. 1981. Observations on the development of the ovarian balls of Moniliformis (Acanthocephala). Parasitology 83: 23–32. doi: 10.1017/S0031182000050009
Aznar, F. J., A. O. Bush, and J. A. Raga. 2002. Reduction and variability of trunk spines in the acanthocephalan Corynosoma cetaceum: The role of physical constraints on attachment. Invertebrate Biology 121: 104–114. doi: 10.1111/ j.1744-7410.2002.tb00051.x
Aznar, F. J., E. A. Crespo, J. A. Raga, and J. S. Hernández-Orts. 2016. Trunk spines in cystacanths and adults of Corynosoma spp. (Acanthocephala): Corynosoma cetaceum as an exceptional case of phenotypic variability. Zoomorphology 135: 19–31. doi: 10.1007/s00435-015-0290-7
Brooks, D. R., and S. M. Bandoni. 1988. Coevolution and relicts. Systematic Zoology 37: 19–33. doi: 10.2307/2413186
Brooks, D. R., and D. A. McLennan. 2002. The Nature of Diversity: An Evolutionary Voyage of Discovery. University of Chicago Press, Chicago, Illinois, United States, 676 p.
Brooks, D. R., and D. A. McLennan. 1993. Parascript: Parasites and the Language of Evolution. Smithsonian Institution Press, Washington, DC, United States, 429 p.
Brooks, D. R., and D. A. McLennan. 1991. Phylogeny, Ecology, and Behavior: A Research Program in Comparative Biology. University of Chicago Press, Chicago, Illinois, United States, 434 p.
Brusca, R. C., and W. Moore. 2016. Invertebrates. Sinauer Associates, Sunderland, Massachusetts, United States, 1,104 p.
Bullock, W. L. 1969. Morphological features as tools and pitfalls in acanthocephalan systematics. In G. D. Schmidt, ed. Problems in Systematics of Parasites. University Park Press, Baltimore, Maryland, United States, p. 9–24.
Coady, N. R., and B. B. Nickol. 2000. Assessment of parenteral Plagiorhynchus cylindraceus (Acanthocephala) infections in shrews. Comparative Parasitology 67: 32–39.
Conway Morris, S., and D. W. T. Crompton. 1982. The origins and evolution of the Acanthocephala. Biological Review 57: 85–115. doi: 10.1111/j.1469-185X.1982.tb00365.x
Crompton, D. W. T., and B. B. Nickol, eds. 1985. Biology of the Acanthocephala. Cambridge University Press, Cambridge, United Kingdom, 519 p.
Crook, J. R., and A. W. Grundmann. 1964. The life history and larval development of Moniliformis clarki (Ward, 1917). Journal of Parasitology 50: 689–693. doi: 10.2307/3276131
Dunagan, T. T., and D. M. Miller. 1991. Acanthocephala. In F. W. Harrison and E. E. Ruppert, eds. Microscopic Anatomy of Invertebrates, Volume 4: Aschelmintes. Wiley, New York, New York, United States, p. 299–332.
Dunagan, T. T., and D. M. Miller. 1978. Anatomy of the genital ganglion of the male acanthocephalan, Moniliformis moniliformis. Journal of Parasitology 64: 431–435. doi: 10.2307/3279775
Dunagan, T. T., and D. M. Miller. 1983. Apical sense organ of Macracanthorhynchus hirudinaceus (Acanthocephala). Journal of Parasitology 69: 897–902. doi: 10.2307/3281054
Dunagan, T. T., and R. Price. 1985. Genital ganglion and associated structures in male Neoechinorhynchus cylindratus (Acanthocephala). Proceedings of the Helminthological Society of Washington 52: 206–209.
Emde, S., S. Rueckert, H. W. Palm, and S. Klimpel. 2012. Invasive Ponto-Caspian amphipods and fish increase the distribution range of the acanthocephalan Pomphorhynchus tereticollis in the River Rhine. PLoS One 7: e53218. doi: 10.1371/journal.pone.0053218
Esch, G. W. 2000. Experimental investigation of physiological factors that may influence microhabitat specificity exhibited by Leptorhynchoides thecatus (Acanthocephala) in green sunfish (Lepomis cyanellus). Journal of Parasitology 86: 685–690. doi: 10.2307/3284948
García-Varela, M., G. Pérez-Ponce de León, P. De la Torre, M. P. Cummings, et al. 2000. Phylogenetic relationship of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. Journal of Molecular Evolution 50: 532– 540. doi: 10.1016/S1055-7903(02)00020-9
Golvan, Y. J. 1994. Nomenclature of the Acanthocephala. Research and Reviews in Parasitology 54: 135–205.
Herlyn, H., N. Martini, and U. Ehlers. 2001. Organisation of the praesoma of Paratenuisentis ambiguus (Van Cleave, 1921) (Acanthocephala: Eoacanthocephala), with special reference to the lateral sense organs and musculature. Systematic Parasitology 50: 105–116. doi: 10.1023/A:1011925516086
Herlyn, H., O. Piskurek, J. Schmitz, U. Ehlers, et al. 2003. The syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. Molecular Phylogenetics and Evolution 26: 155–164. doi: 10.1016/S1055-7903(02)00309-3
Leadabrand, C. C., and B. B. Nickol. 1993. Establishment survival, site selection and development of Leptorhynchoides thecatus in largemouth bass, Micropterus salmoides. Parasitology 106: 495–501. doi: 10.1017/ S0031182000076794
Lorenti, E., S. M. Rodríguez, F. Cremonte, G. D’Elía, et al. 2018. Life cycle of the parasite Profilicollis chasmagnathi (Acanthocephala) on the Patagonian coast of Argentina based on morphological and molecular data. Journal of Parasitology 104: 479–485. doi: 10.1645/17-134
Maggenti, M. A. B., A. R. Maggenti, and S. L. Gardner. 2017. Dictionary of Invertebrate Zoology. Zea Books, Lincoln, Nebraska, United States, 982 p. doi: 10.13014/K2DR2SN5
Miller, D. M., and T. T. Dunagan. 1985a. Functional morphology. In D. W. T. Crompton and B. B. Nickol, eds. Biology of the Acanthocephala. Cambridge University Press, Cambridge, United Kingdom, p. 73–123.
Miller, D. M., and T. T. Dunagan. 1985b. New aspects of acanthocephalan lacunar system as revealed in anatomical modeling by corrosion cast method. Proceedings of the Helminthological Society of Washington 53: 221–226.
Miller, D. M., and T. T. Dunagan. 1983. A support cell to the apical and lateral sensory organs in Macracanthorhynchus hirudinaceus (Acanthocephala). Journal of Parasitology 69: 534–538. doi: 10.2307/3281367
Miller, D. M., and T. T. Dunagan. 1984. A support cell to the apical and lateral sensory organs in Moniliformis moniliformis (Acanthocephala). Proceedings of the Helminthological Society of Washington 51: 221–224.
Monks, S. 2001. Phylogeny of the Acanthocephala based on morphological characters. Systematic Parasitology 48: 81–116. doi: 10.1023/A:1006400207434
Monks, S., and G. Pérez-Ponce de León. 1996. Koronacantha mexicana n. gen., n. sp. (Acanthocephala: Illiosentidae) from marine fishes in Chamela Bay, Jalisco, México. Journal of Parasitology 82: 788–792. doi: 10.2307/3283892
Monks, S., and D. J. Richardson. 2011. Phylum Acanthocephala Kohlreuther, 1771. In Z.-Q. Zhang, ed. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Magnolia Press, Auckland, New Zealand, p. 234–237. https://www.mapress.com/ zootaxa/2011/f/zt03148p237.pdf
Monks, S., B. Alemán-García, and G. Pulido-Flores. 2008. A new species of Dollfusentis Golvan, 1969 (Palaeacanthocephala: Illiosentidae) in the striped mojara, Eugerres plumieri (Perciformes: Actinoptergii), from Bahía de Chetumal, Quintana Roo, México. Zootaxa 1853: 45–56. https:// repository.uaeh.edu.mx/bitstream/handle/123456789/7559
Monks, S., F. Marques, V. León-Règagnon, and G. Pérez- Ponce de León. 1997. Koronacantha pectinaria n. comb. (Acanthocephala: Illiosentidae) from Microlepidotus brevipinnis (Haemulidae) and redescription of Tegorhynchus brevis. Journal of Parasitology 83: 485–494. doi: 10.2307/3284415
Monks, S., G. Pulido-Flores, and J. Violante-González. 2011. A new species of Neoechinorhynchus (Acanthocephala: Neoechinorhynchidae) in Dormitator latifrons (Perciformes: Eleotridae) from the Pacific Coast of Mexico. Comparative Parasitology 78: 21–28. doi: 10.1654/4462.1
Moore, D. V. 1946. Studies on the life history and development of Moniliformis dubius Meyer, 1933. Journal of Parasitology 32: 257–271. doi: 10.2307/3272873
Muñoz, G., and M. George-Nascimento. 2002. Spiracanthus bovichthys n. gen. n. sp. (Acanthocephala: Arhythmacanthidae), a parasite of littoral fishes of the central south coast of Chile. Journal of Parasitology 88: 141–145. doi: 10.2307/3285405
Near, T. J., J. R. Garey, and S. A. Nadler. 1998. Phylogenetic relationships of the Acanthocephala inferred from 18s ribosomal DNA sequences. Molecular Phylogenetics and Evolution 10: 287–298. doi: 10.1006/mpev.1998.0569
Nickol, B. B. 1985. Epizootiology. In D. W. T. Crompton and B. B. Nickol, eds. Biology of the Acanthocephala. Cambridge University Press, Cambridge, United Kingdom, p. 307–346.
Patil, H. 2022. Pomphorhynchus laevis. Alchetron. https:// alchetron.com/Pomphorhynchus-laevis
Petrochenko, V. I. 1956. [Acanthocephala of Domestic and Wild Animals, Volume I.] Izdatel’stvo Akademii Nauk SSSR, Vsesiuznoe Obshchestvo Gel’mintologov, Moscow, Soviet Union, 465 p. [In Russian.]
Petrochenko, V. I. 1958. [Acanthocephala of Domestic and Wild Animals, Volume II.] Izdatel’stvo Akademii Nauk SSSR, Vsesiuznoe Obshchestvo Gel’mintologov, Moscow, Soviet Union, 435 p. [In Russian.]
Pritchard, M. H., and G. O. W. Kruse. 1982. The collection and preservation of animal parasites. Technical Bulletin 1. Harold W. Manter Laboratory and University of Nebraska Press, Lincoln, Nebraska, United States, 141 p.
Richardson, D. J., and B. B. Nickol. 1999. Physiological attributes of the pyloric caeca and anterior intestine of green sunfish (Lepomis cyanellus) potentially influencing microhabitat specificity of Leptorhynchoides thecatus (Acanthocephala). Comparative Biochemistry and Physiology, Part A 122: 375–384. doi: 10.1016/S1095-6433(99)00012-4
Richardson, D. J., and K. E. Richardson. 2009. Transmission of paratenic Leptorhynchoides thecatus (Acanthocephala) from green sunfish (Lepomis cyanellus) to largemouth bass (Micropterus salmoides). Comparative Parasitology 76: 290–292. doi: 10.1654/4395.1
Richardson, D. J., S. Monks, M. García-Varela, and G. Pulido-Flores. 2010. Redescription of Centrorhynchus microcephalus (Bravo-Hollis, 1947) Golvan, 1956 (Acanthocephala: Centrorhynchidae) from the groove- billed ani (Crotophaga sulcirostris) in Veracruz, Mexico. Comparative Parasitology 77: 164–171. doi: 10.1654/4412.1
Richardson, K. E., D. J. Richardson, and B. B. Nickol. 2008. Emigration of Leptorhynchoides thecatus (Acanthocephala) in green sunfish (Lepomis cyanellus). Comparative Parasitology 75: 49–51. doi: 10.1654/4296.1
Sielaff, M., H. Schmidt, T. H. Struck, D. Rosenkranz, et al. 2016. Phylogeny of Syndermata (syn. Rotifera): Mitochondrial gene order verifies epizoic Seisonidea as sister to endoparasitic Acanthocephala within monophyletic Hemirotifera. Molecular Phylogenetics and Evolution 96: 79–92. doi: 10.1016/j.ympev.2015.11.017
Tavares dos Santos, V. G., and S. B. Amato. 2010. Rhinella fernandezae (Anura, Bufonidae) a paratenic host of Centrorhynchus sp. (Acanthocephala: Centrorhynchidae) in Brazil. Revista Mexicana de Biodiversidad 81: 53–56. http:// www.scielo.org.mx/PDF/rmbiodiv/v81n1/v81n1a8.PDF
Van Cleave, H. J. 1949. Morphological and phylogenetic interpretations of the cement glands in the Acanthocephala. Journal of Morphology 84: 427–457. doi: 10.1002/ jmor.1050840304
Weber, M., A. R. Wey-Fabrizius, L. Podsiadłowski, A. Witek, et al. 2013. Phylogenetic analyses of endoparasitic Acanthocephala based on mitochondrial genomes suggest secondary loss of sensory organs. Molecular Phylogenetics and Evolution 66: 182–189. doi: 10.1016/j. ympev.2012.09.017
Wiley, E. O., D. Siegel-Causey, D. R. Brooks, and V. A. Funk. 1991. The Compleat Cladist: A Primer of Phylogenetic Procedures. University of Kansas, Lawrence, Kansas, United States, 158 p. doi: 10.5962/bhl.title.4069
Wright, R. D. 1970. Surface ultrastructure of the acanthocephalan lemnisci. Proceedings of the Helminthological Society of Washington 37: 52–56.
Yamaguti, S. 1963. Systema Helminthum, Volume V: Acanthocephala. Interscience, New York, New York, United States, 423 p.

