10 Chapter 10 – Introduction to the Nematomorphs
Introduction
Nematomorphs are commonly known as horsehair worms because of their resemblance to and the common myth of worms arising from horse tail or mane hairs that fall into water. Additionally, because of their habit of becoming entan- gled in masses of many individuals while mating, horsehair worms are also known as Gordian worms after the Gordian knot from Greek mythology (Figure 1) (Bolek et al., 2015).

Figure 1. Free-living adult gordiids. A) Adult free-living male Gordius sp.; B) A typical Gordian knot containing numerous in- dividuals of G. terrestris. Source: M. G. Bolek. License: CC BY- NC-SA 4.0.
The phylum Nematomorpha consists of species that can be allocated into 2 major classes, the freshwater and terres- trial Gordiida and the marine Nectonematida. These animals are unique in several ways and they are 1 of 3 entirely parasitic animal phyla that include the Cestoda—the tapeworms— and the Acanthocephala—the thorny-headed worms (Hanelt et al., 2005). At the current time, the nematomorphs include approximately 360 species that have been described globally and are included in 19 extant and 2 extinct genera (Poinar, 1999; Poinar and Buckley, 2006; Yadav et al., 2018). The 5 known marine species belong to the genus Nectonema, and all infect decapod crustaceans (phylum Crustacea, class Decapoda) (see Schmidt-Rhaesa, 2013).
Among the order Gordiida, both dioecious and parthenogenetic species are known and at least 1 species occurs in terrestrial habitats (Hanelt et al., 2012; Anaya et al., 2019). The freshwater and terrestrial gordiids have complex life cycles, which means that the life cycle can be completed by using multiple hosts with final free-living larvae and adults. Species of gordiids that infect an insect such as a cricket, appear to influence the cricket to go near or into water where the adult worm then emerges from the insect to continue its life in a free-living phase (Thomas et al., 2002; 2003). After emerging from their host, dioecious species form large mating assemblages, also called Gordian knots, where they mate and females deposit egg strings on substrate in the water. Those species that are parthenogenetic immediately deposit egg strings after emerging from their host (Hanelt et al., 2012; Bolek et al., 2013a). Larvae develop in the water and infect various species of aquatic invertebrate animals (Bolek and Coggins, 2002; Hanelt and Janovy, 2003). Some of these infected animals (such as aquatic insect larvae) act as paratenic or transport hosts and when the insects metamorphose they can carry the cysts to a terrestrial environment where they may be consumed by omnivorous or predatory arthropods, including millipedes, orthopterans (crickets, grasshoppers, etc.), beetles, cockroaches, and mantids (Figure 2).
Horsehair worms are commonly found in domestic water sources such as swimming pools, toilet bowls, cow troughs, pet water bowls, and more, thus making human interactions with them quite common (Bolek, 2000; Hanelt et al., 2005). However, besides the trauma people experience when they discover nematomorphs in their toilet or pet’s water bowl, they have no medical or economic importance, although their potential as biological control agents has been suggested (Schmidt-Rhaesa, 2013). There are a few reports of adult horsehair worms from humans, but all of these observations are most likely the result of people swallowing infected arthropods or arthropod hosts releasing free-living worms into drinking water (Bolek et al., 2015). Additionally, there is one odd report of larval horsehair worms in human facial tissue resulting in orbital tumors (Singh and Rao, 1966). However, this report is questionable because juvenile worms contain few if any morphological characteristics of gordiids (see Schmidt-Rhaesa, 2013).
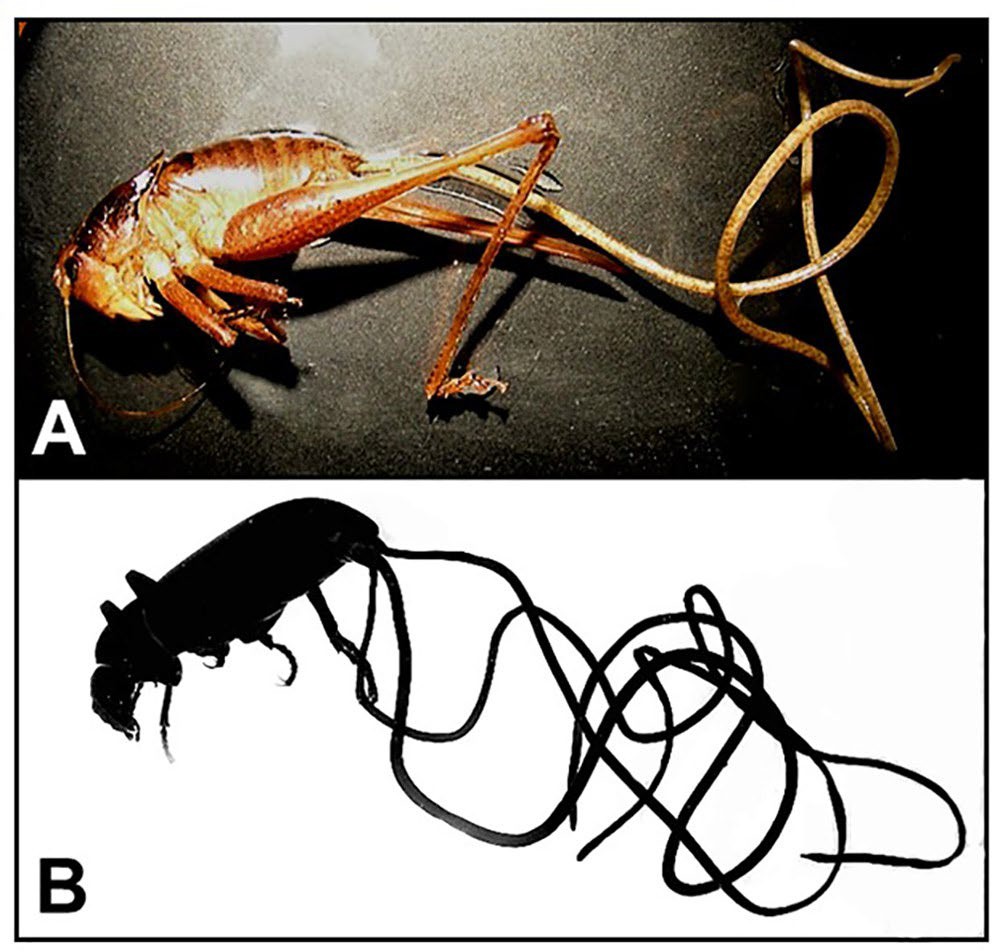
Figure 2. Examples of typical arthropod definitive hosts for gordiids. A) An undescribed species of shieldback katydid Atlanticus sp. with an emerging female Chordodes morgani; B) an unidentified tenebrionid beetle with 3 emerging individuals of a new species of Parachordodes. Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Chief Morphological Characters
The phylum Nematomorpha belongs to the superphylum Ecdysozoa. As with all ecdysozoans (which means molting animals), horsehair worms molt their cuticle at least once during their life history. As free-living adults, nematomorphs are very long, cylindrical, and thin, and range from a few cm to over 2 m in length and less than 1 to 3 mm in diameter (Bolek et al., 2015). However, most free-living worms are 20–40 cm in length (Bolek and Coggins, 2002; Schmidt- Rhaesa, 2013).
Among the freshwater and terrestrial gordiids, adult females are usually longer and thicker than adult males (Bolek and Coggins, 2002). Adult free-living worms vary in color from white to shades of dark brown. In some species of Chordodes, some individuals have dark patches on a lighter background, producing a leopard-skin pattern, whereas in species of the genus Gordius some individuals may have white spots on a darker background (Figure 3C, D). However, color is not a good characteristic for species identification, and most species for which information is available contain various color morphs within and among populations (Schmidt-Rhaesa, 2013).
The anterior end in free-living adults is spheroid or disinctly tapering (Figure 3E, F). The mouth may be visible or closed (Figure 3H), and no other structures are present on the anterior end. In some species, there is a distinctly lighter colored area on the anterior end known as a calotte, followed by a darkly pigmented ring (Figure 3G). The posterior end is tri-lobed or unbranched in females and bi-lobed or unbranched in males (Figure 4A–D). In males, the cloacal opening is always situated on the ventral side and may contain cuticular structures, such as circumcloacal spines. The cloaca of males is usually surrounded by either post-cloacal crescents or spines and/or pre-cloacal bristles, and these structures are genus- and/or species-specific (Figure 4C). The cloaca in females is terminal or slightly subterminal and circumcloa- cal spines have not been reported for females of most spe- cies (Bolek and Coggins, 2002; Schmidt-Rhaesa et al., 2003; Bolek et al., 2010; Begay et al., 2012; Schmidt-Rhaesa, 2013). The marine Nectonema species are morphologically similar to the gordiids, wormlike, long, 10–270 mm for males and 30–960 mm for females and approximately 1 mm in diameter (Schmidt-Rhaesa, 2013). The anterior and posterior ends are rounded in females, whereas the posterior end is curved ventrally and distinctly tapered in males. Unlike most gordiids, the cuticle is smooth and does not contain areoles or other surface structures, but instead the dorsal and ventral longitudinal midlines contain natatory bristles (Figure 5). In addition to the ventral longitudinal nerve cord, a dorsal nerve cord is present. The intestine is incomplete and forms a blind gut. Unlike the freshwater gordiids, the anterior end of nectonematids contains a body cavity with conspicuous large cells of unknown function known as giant cells. Additional work by Schmidt- Rhaesa (1996a; 1996b) and Restelli and colleagues (2002) indicates that gordiids and nectonematids differ in their muscle cell structure. Freshwater gordiids have thick and thin contractile filaments which are concentrated in bundles as thick sheets and myofibrils enclose the cell body; whereas nectonematid muscle cells are coelomtyarian, as in some nematodes.
General larval morphology: Larvae are 60–100 μm by 14–30 μm in length and width, respectively, cylindrical in shape, and superficially annulated. A septum divides the larval body into 2 regions, the pre-septum and the post-sep- tum (Figure 6). The pre-septum contains 3 rings of cuticular hooks and an eversible proboscis, supported by 3 internal stylets and various sets of muscles (Müller et al., 2004). The outer cuticular ring contains 6 hooks, 1 of which is positioned ventrally and bifurcated; whereas the middle and inner rings contain 6 hooks, none of which is bifurcated (Szmygiel et al., 2014). The post-septum contains 1–4 terminal spines among some gordiid genera (Szmygiel et al., 2014). Internally, the post-septum contains the pseudointestine, which is subdivided into unequal portions and opens to the outside of the body via a small duct (Hanelt and Janovy, 2002; Szmygiel et al., 2014). The pseudointestine is assumed to have a glandular function and empties during cyst formation.
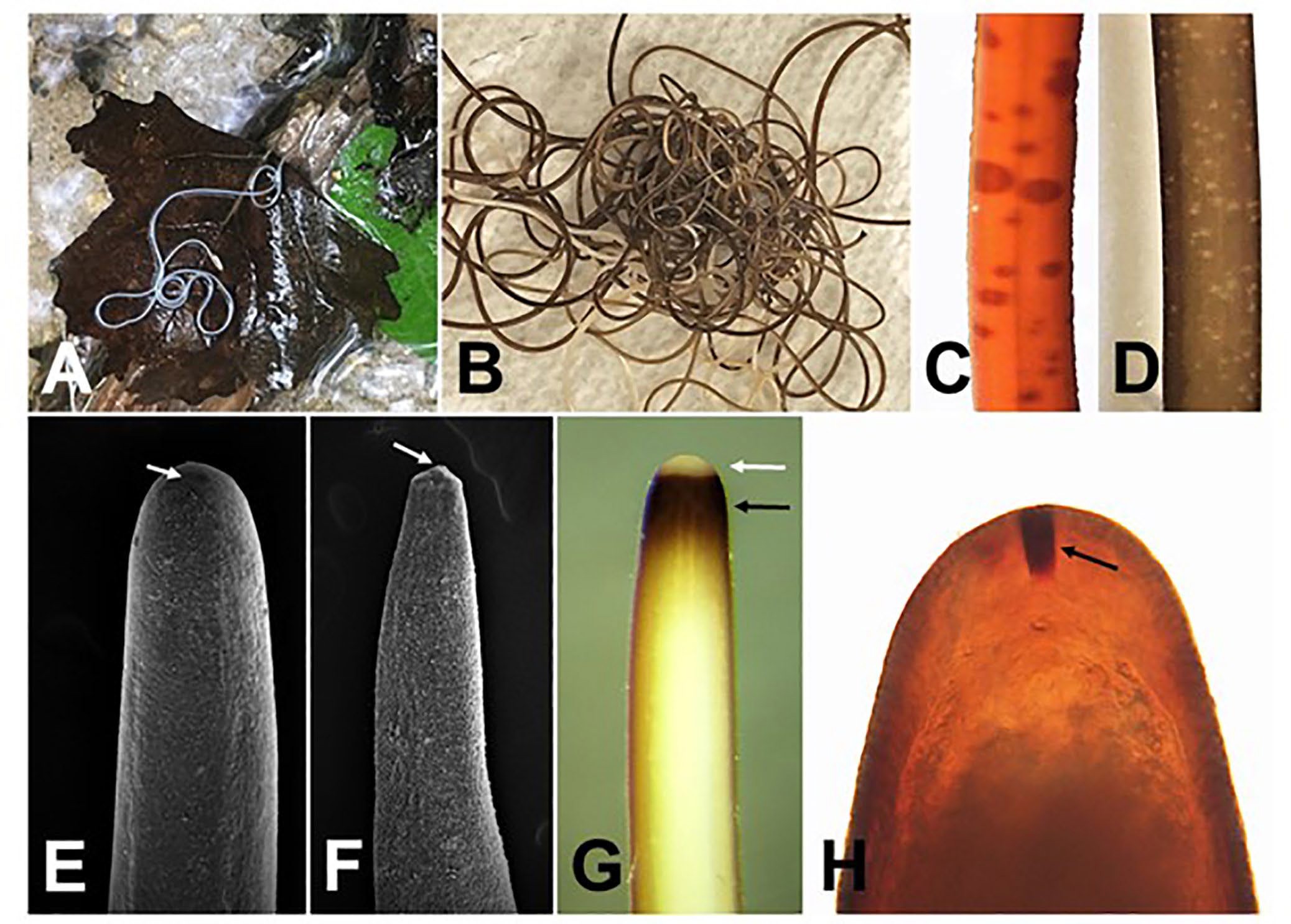
Figure 3. Color and anterior morphology of adult free-living gordiids. A) Typical white color of a female Gordius difficilis; B) a Gordian knot of G. terrestris showing variation in color of worms ranging from white to dark brown; C, D) mid-body region of a free-living adult female Chordodes morgani showing the leopard pattern and the mid-body region of a free-living adult male G. terrestris showing white spots on a darker background; E, F) scanning electron micrograph of the E) anterior region being spherical in G. difficilis and F) distinctly tapering in C. morgani. Note the degenerate mouth (white arrows); G) anterior end of a female G. terrestris. Note the calotte (white arrow) followed by a by a dark pigmented ring (black arrow); H) anterior end of a male C. morgani. Note the cuticularized pharynx (black arrow). Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Cuticular Features
In most species of nematomorphs, the surface of the cu- ticle is smooth or structured into elevated thickenings called areoles. When present these are separated by interareolar furrows; in addition, a variety of short spines and/or bristles may be present on the surface of the cuticle. In most gordiid species, 1 or 2 types of areoles are present. These are known as simple areoles that form a regular pattern on the cuticle (Fig- ure 4F). In species of some genera, such as those in the genus Gordius, areoles are lacking or are weakly developed, while in species of other genera, such as those in the genus Chordodes, up to 6 different types of areoles can be present and include the characteristic crowned areoles which define the species in this genus (Figure 4I–J) (Schmidt-Rhaesa et al., 2003; Bolek et al., 2013b). Finally, in some species, 2 or more areoles may be fused and form structures referred to as mega-areoles and super-areoles, and these are considered synapomorphies that group species into defined genera (Figure 4G, H).
Sexual dimorphism is common in the areole pattern among gordiids. For proper descriptions and to make firm identifications to the level of species, it is necessary to have examples of and describe the characters of both sexes for complete species descriptions (Bolek and Coggins, 2002; Bolek et al., 2010; 2013b).
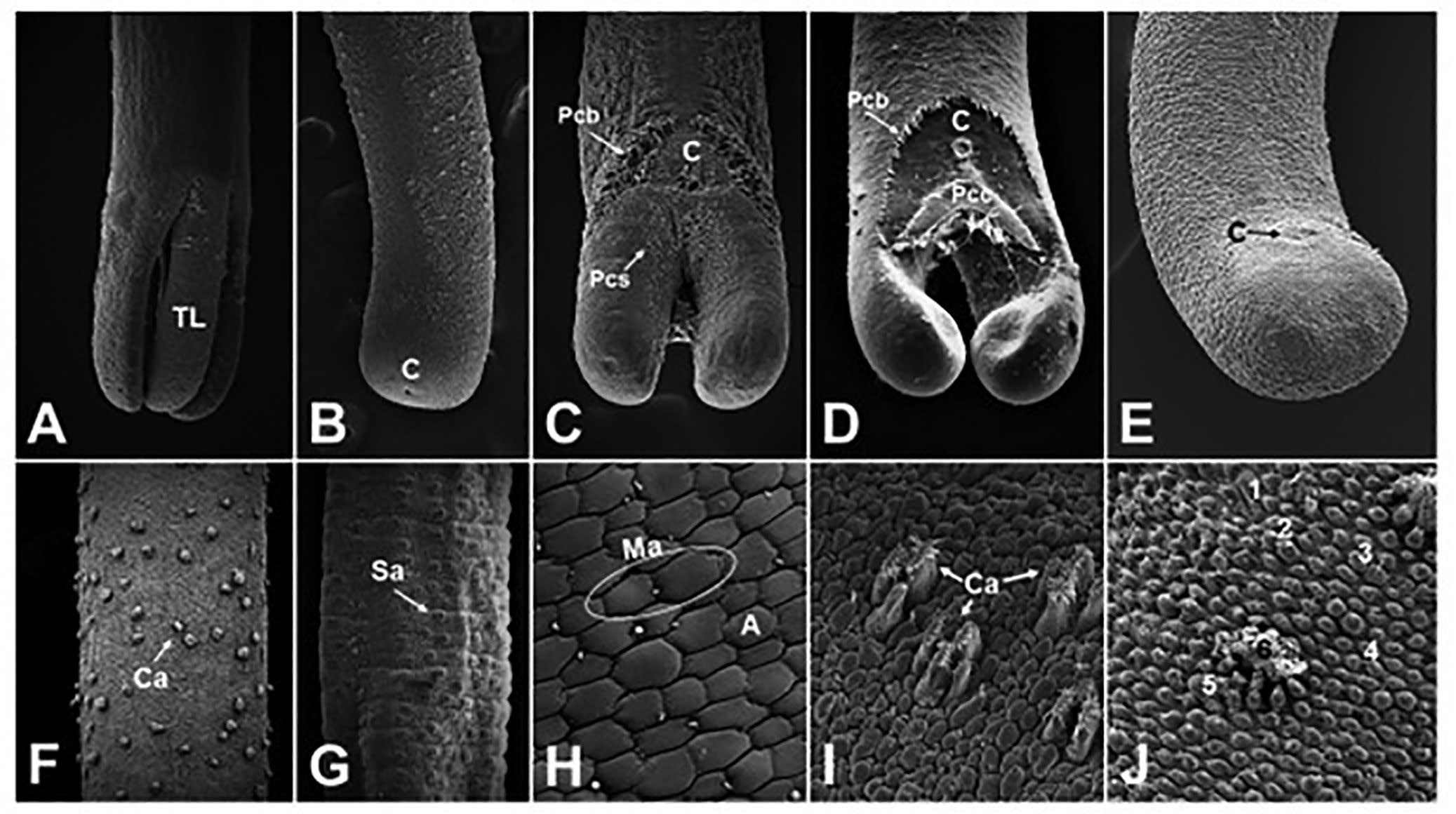
Figure 4. Scanning electron micrographs of the posterior ends and cuticle structures of adult free-living gordiids. A) Posterior end of a female Paragordius sp. Note the 3 tail lobes (TL); B) posterior end of a female Chordodes sp. showing a round posterior end and a terminal cloaca (C); C) posterior end of a male Parachordodes sp. showing 2 tail lobes, cloacal spines surrounding the ventrally located cloaca (C) and rows of precloacal bristles (Pcb), and postcloacal spines (Pcs); D) posterior end of a male G. difficilis. Note the ventrally located cloaca (C), precloacal bristles (Pcb), and postcloacal crescent (Pcc); E) a male Neochordodes sp. Note the absence of distinct tail lobes and the cloacal opening (C); F) mid-body region of an adult free-living female Chordodes sp. Note the distinct crown areoles (Ca) among simple areoles; G) mid-body region of an adult free-living male Parachordodes sp. showing characteristic super-areoles (Sa); H) higher magnification of the mid-body cuticle of a male Neochordodes sp. Note the simple (A) and mega-areoles (Ma); I, J) higher magnification of the mid-body cuticle of 2 species of Chordodes showing interspecies variation in crown areoles (Ca). Note the 5 types of areoles (1–5) on the cuticle of the Chordodes species in (J). Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Body Wall
The body wall of adult gordiids is composed of a thick cuticle containing an outer homogeneous region and an inner fibrous region. The fibrous region consists of 25–45 layers of thick fibrils that are arranged in a crisscross pattern alternating at an angle of 60–65° (May, 1919; Schmidt-Rhaesa, 1997; 2013). Studies on the chemical composition of the cuticle by Brivio and colleagues (2000) and Protasioni and colleagues (2003) indicate that the makeup of the fibrils is not collagen but some other proteinaceous components. Below the cuticle is a very thin epidermis, which secretes the cuticle lay- ers during development within the definitive host (Schmidt- Rhaesa, 2013). The musculature in all nematomorphs consists of longitudinal muscles and, as in species of the phylum Nemata, circular muscles are absent (Schmidt-Rhaesa, 1996a; 1996b; Restelli et al., 2002). The body cavity in free-living adults is mostly filled with gonads and vacuolated parenchyma cells filled with lipids and glycogen, and the digestive track is greatly reduced (Reutter, 1972).
Nervous System
The nervous system consists of a brain (basically a circumesophageal nerve commisure), a ventral longitudinal nerve cord, which emerges from the ventral part of the brain, and a number of peripheral basi-epidermal nerves. The brain forms a ring-like structure similar to those possessed by species in the phylum Nemata and surrounds the anterior region of the alimentary canal (Schmidt-Rhaesa, 2013). The ventral nerve cord is connected to the epidermis by a thin lamella (Schmidt-Rhaesa, 1997). Simple sensory organs in the cuticle are not fully understood, but some studies indicate that integumentary receptors are present. However, it is unclear if these function as mechanoreceptors or if they have other functions (Schmidt-Rhaesa, 2013).
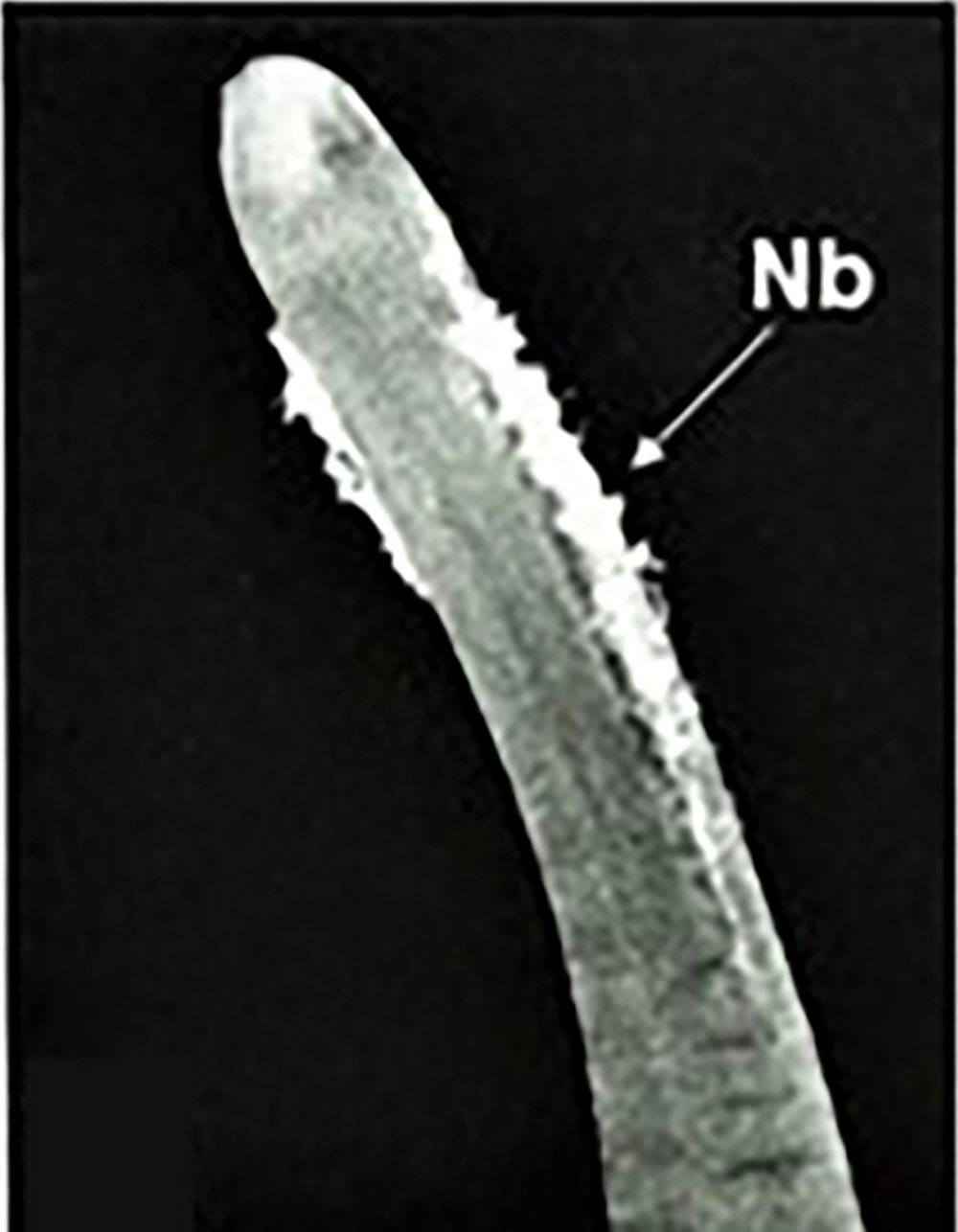
Figure 5. Light micrograph showing natatory bristles (Nb) on the anterior part of a mature fee-living gordiid. Source: M. G. Bolek. License: CC BY-NC-SA 4.0
Digestive System
In free-living adults, a mouth may or may not be present and, depending on the gordiid species, the pharynx can be a cuticularized tube (Figure 3H), cellular in structure, or absent altogether. The intestine is located dorsally to the ven- tral nerve cord and consists of layers of cuboidal cells that manifest microvilli on the lumen side of the tissue. Work on Paragordius varius indicates that the organization of the intestine changes during development in the definitive host, decreasing in free-living adults compared to parasitic juveniles. In both sexes, the intestine and reproductive system fuse and form the cloaca, which is lined with cuticle (Schmidt-Rhaesa, 1997; 2005; 2013).
Reproductive System
The gonads are arranged as 2 long dorsolateral tubes, surrounded by parenchymal cells, and extend almost the entire length of the body. In mature males, the 2 testes are full of spermatozoa but may be empty after males complete mating with several females. In developing females, the 2 dorsolateral tubes contain ovarial ducts with numerous extensions called ovaries (Schmidt-Rhaesa, 1997).
Little information is available about the structure of gonads in the marine Nectonema species, including no informa- tion on mature spermatozoa (Schmidt-Rhaesa, 2013).
Reproduction
Once worms enter water, in dioecious species, male and female worms must find each other to mate. Observations on Paragordius varius in the laboratory indicate that male worms begin mating with females even before they com- pletely exit their arthropod definitive host (Hanelt and Janovy, 2004b). However, field studies indicate that most arthropods that are shown to be infected by horsehair worm larvae are infected with a single worm and these individuals must some-how find the opposite sex for copulation after they emerge from their hosts (Bolek and Coggins, 2002; Looney et al., 2012). Currently, it is unknown whether both sexes find each other by the use of attractants, such as pheromones, or simply by chance. However, field studies using daily collections of individual female Gordius difficilis indicate that most females mate within a day of emerging from their host (Bolek and Coggins, 2002).
Both field and laboratory observations indicate that male and female worms initiate typical Gordian knots within hours to days of being placed together (Bolek and Coggins, 2002; Bolek et al., 2013b). During mating, males move up and down the female’s body with their coiled posterior end. In genera with bi-lobed posterior ends, such as Gordius, a male will spread its tail lobes and glide along the female’s body (Figure 7). Once a male’s cloaca is in proximity of the female cloaca, the male deposits a mass of sperm referred to as a sperm drop or spermatophore (Figure 7). Field studies on Gordius difficilis indicate that sperm drops can remain on the posterior region of females for at least a week (Figure 7) (Bolek and Coggins, 2002).
The spermatozoa of gordiids are unique and change shape during sperm transfer between a male and a female. They contain a nucleus and compartments, which have been named the acrosomal tube, acrosomal sheath, and multivesicular complex (Figure 7). It is unclear if and how these spermatozoa move because they lack a flagellum or pseudopods (Schmidt-Rhaesa, 2013).
After dioecious species mate, females produce up to 8 million eggs during their 2-week to 2-month adult life span (Bolek and Coggins, 2002; Hanelt, 2009). Females in the genus Gordius and Acutogordius deposit short pieces of egg strings approximately 1–2 cm in length on the substrate or while within Gordian knots. In contrast, females of spe- cies of Euchordodes, Chordodes, and Neochordodes deposit their egg strings in a zigzag pattern to objects such as sticks or rocks in water. Finally, females of Paragordius species deposit a single long egg string approximately 1–5 times the length of the worm’s body in the water column and/or in algal mats (Figure 8) (Poinar, 2010; Szmygiel et al., 2004; Bolek et al., 2015; Chiu et al., 2017).
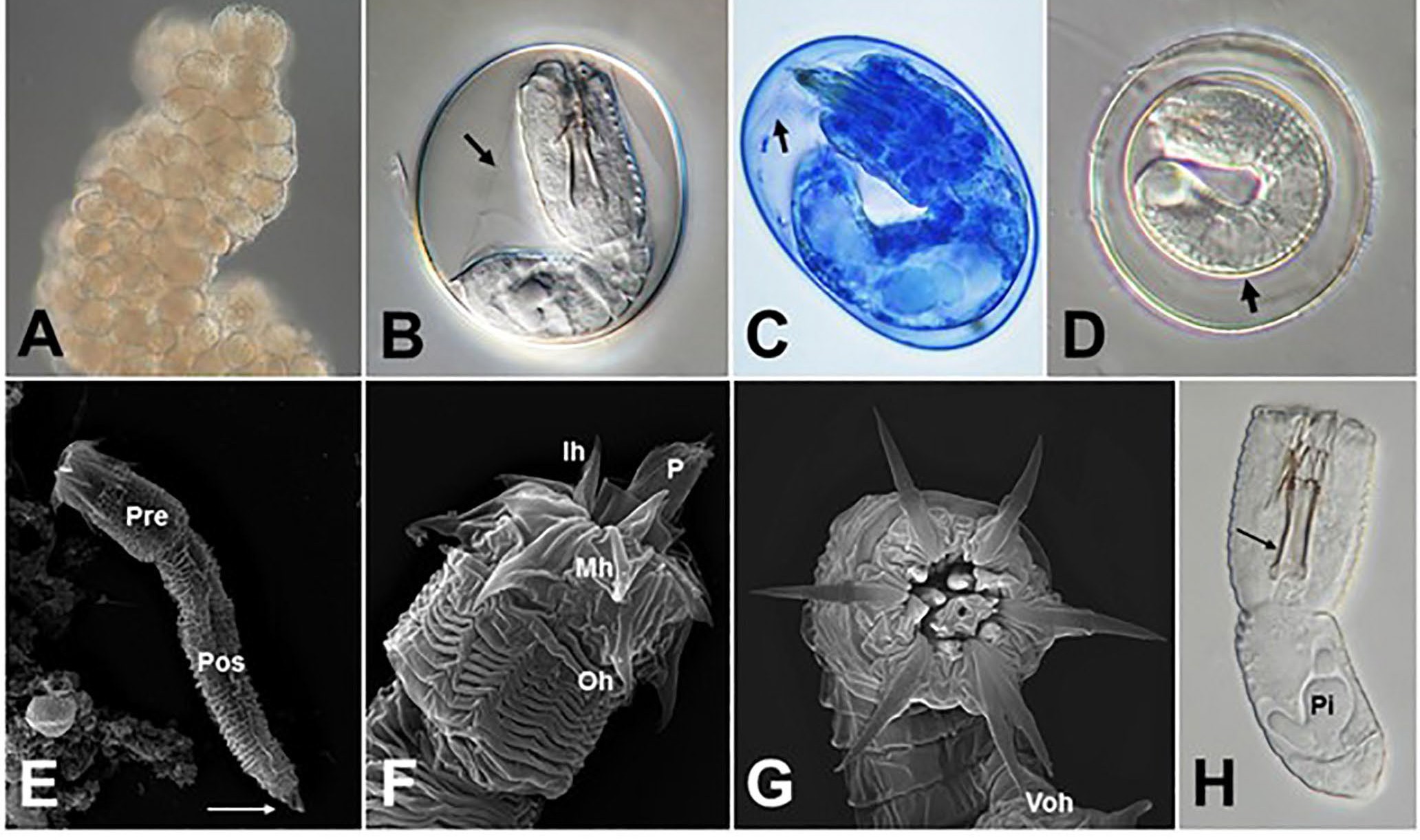
Figure 6. Eggs and larvae of gordiids. A) Higher magnification of pieces of Gordius difficilis egg strings showing the concentration of eggs; B, C) typical eggs of freshwater gordiids. Note the developed larva surrounded by a thin inner membrane (arrow) in each egg; D) an unusual egg of G. terrestris, a gordiid that lays eggs in the soil. Note the outer shell separated by distinct space from a thick inner membrane (arrow) surrounding the larva; E) scanning electron micrograph of a typical Gordius larva. Note the pre-septum (Pre), post-septum (Pos), and terminal spine (arrow) on the post-septum; F) scanning electron micrograph of the anterior end of a Gordius larva. Note the proboscis (P) and 3 rings of cuticular hooks including the inner, middle, and outer hooks (Ih, Mh, Oh); G) scanning electron micrograph of the anterior end of a Paragordius varius larva. Note the dorsoventrally compressed proboscis in relationship to the ventral outer hooks (Voh); H) larva of Chordodes kenyaensis. Note the 3 internal stylets (arrow), and V-shaped pseudointestine (Pi). Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
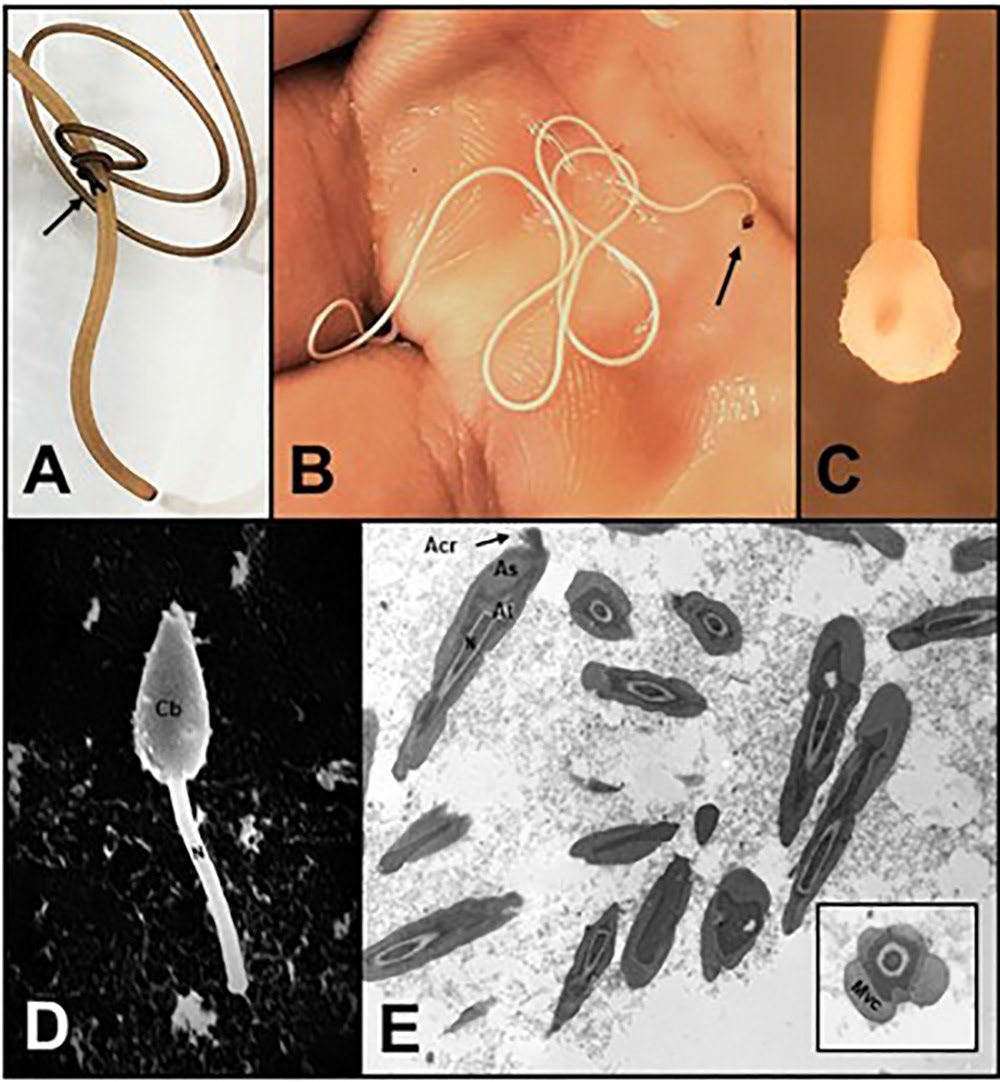
Figure 7. Mating behavior and the morphology of the sperm drop and sperm of gordiids. A) A male Gordius terrestris in the process of initiating mating with a female. Note the thinner size and bi-lobed posterior end (arrow) of the male; B) a field-collected female G. difficilis with a sperm drop (arrow) on the posterior end; C) a higher magnification of the posterior end of a female Paragordius varius with a deposited sperm drop; D) a single sperm on the posterior region of the cloaca of a female G. difficilis. Note the round end (Cb) and rod-shaped end (N) where part of the nucleus is located; E) cross- and longitudinal sections of a spermatozoon from the reproductive system of a Gordius sp. Note the numerous compartments and organelles, including Acr = acrosome, As = acrosomal sheath, At = acrosomal tube, Mvc = multivesicular complex, and N = nucleus. Sources: A–C) M. G. Bolek; D) Bolek and Coggins, 2002; E and insert) A. Schmidt-Rhaesa. License for all: CC BY-NC-SA 4.0.
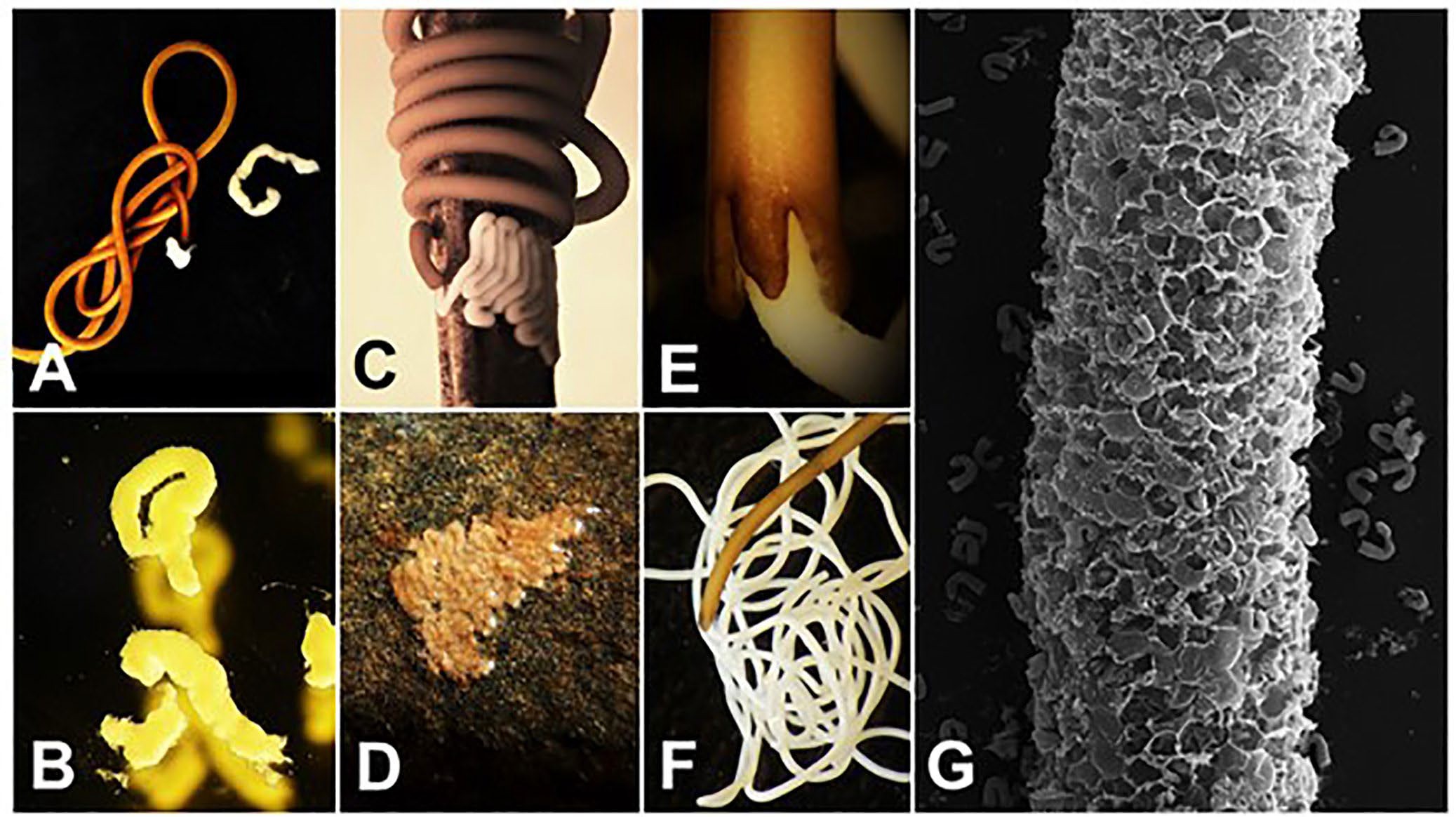
Figure 8. Examples of gordiid egg strings and eggs. A) A female Gordius terrestris (brown) in the process of depositing short pieces of egg strings (white); B) higher magnification of pieces of G. terrestris egg strings; C) a female Chordodes kenyaensis in the process of laying an egg string on a stick. Note the zigzag pattern of the egg string; D) egg string of Chordodes sp. deposited on a rock in a zigzag pattern; E) posterior end of a female Paragordius varius showing an egg string (white) excreted from between the 3 tail lobes; F) posterior end of a female P. varius (tan) in the process of depositing a single and very long egg string (white); G) a scanning electron micrograph of a partial egg string of P. varius with hatched larvae scattered around the periphery of the egg string. Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Eggs are elliptical to round in shape, with a distinct shell and a thin inner membrane surrounding the developing larva. This inner membrane is relatively thin in aquatic species but is much thicker in gordiids that reproduce in terrestrial habitats (Figure 6) (Anaya et al., 2019). After hatching, the free-living gordiid larvae are semi-sessile and not capable of moving great distances.
In order for aquatic gordiid larvae to reach their terrestrial arthropod hosts, 3 transmission strategies have been proposed. These include: 1) Direct consumption of larvae by the definitive hosts while drinking water; 2) larvae encysting on vegetation/detritus and being ingested accidently while the definitive host ingests vegetation/detritus; and 3) larvae entering and encysting within a paratenic host, which is preyed on or scavenged by the definitive hosts (Hanelt et al., 2005; Bolek et al., 2015). A number of studies show that when definitive arthropod hosts ingest suspended larvae when they drink water, the definitive host becomes infected (May, 1919; Inoue, 1962; Hanelt and Janovy, 2004b). However, comparative work by Inoue (1962) and Hanelt and Janovy (2004a) strongly suggests that prevalence and intensities of these infections in definitive arthropod hosts are much lower compared to those that occur when definitive hosts are exposed to gordiid cysts in paratenic hosts. Finally, observations on gordiid larvae of European and North American Gordius species and Asian Acutogordius species indicate that these larvae can encyst freely on the surface of aquatic vegetation, or within the egg strings deposited in the environment, but the role of these cysts in transmission is unclear (Dorier, 1930; Bolek et al., 2015; Chiu et al., 2017).
Studies on larvae of other gordiid species indicate that larvae of these species never encyst on vegetation or de- tritus (May, 1919; Inoue, 1960; Hanelt and Janovy, 2002; Bolek et al., 2010; Hanelt et al., 2012; Bolek et al., 2013a; Szmygiel et al., 2014). In fact, most reports of gordiid cysts have been reported from aquatic metazoan animals including molluscs, annelids, arthropods, fish, and amphibians (Harkins et al., 2016; Chiu et al., 2016; Yamashita et al., 2017). More importantly, experimental studies by Hanelt and Janovy (2004a) demonstrate that 3 phylogenetically distinct species of gordiids indiscriminately infect and form cysts in a variety of aquatic invertebrates and fish. These authors also demonstrated that within non-biting midge paratenic hosts, gordiid cysts survived metamorphosis of these aquatic insects, and when these insects were fed to crickets, the crickets became infected and released adult worms. Taken together, the numerous reports of gordiid cysts infecting a variety of aquatic animals, their ability to survive insect metamorphosis and their ability to infect terrestrial arthropod hosts, supports the paratenic host strategy in the life cycles of gordiids.
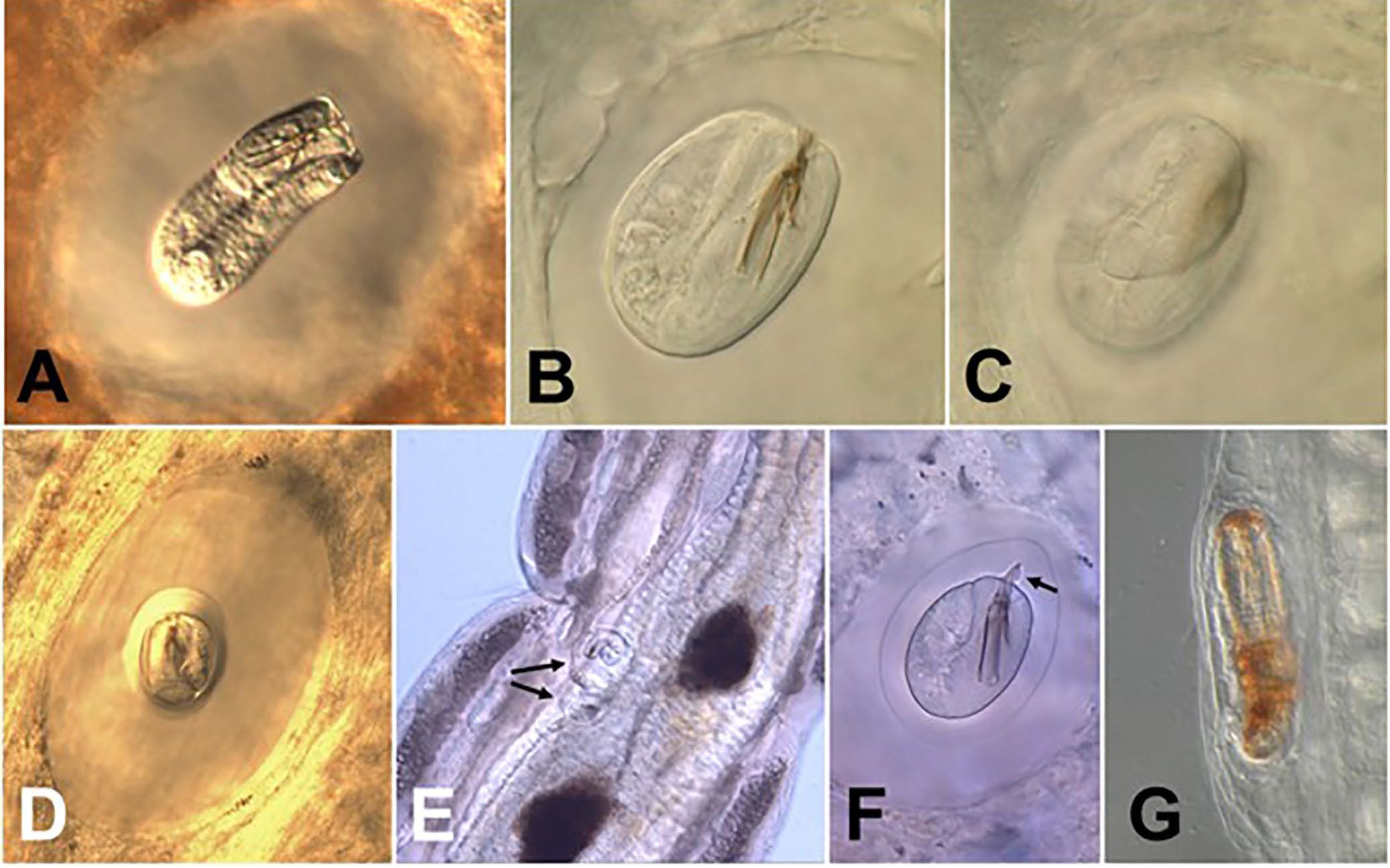
Figure 9. Cysts of gordiids. A) A larval Gordius terrestris in the process of folding into a cyst in its earthworm paratenic host; B, C) typical Gordius type cyst, not the folding pattern of the larva; D) fully developed Gordius type cyst. Note the clear halo-like structure surrounding the folded larva; E) 2 types of gordiid cysts (arrows) in the hemocoel of a non-biting midge larva; F) a Paragordius varius cysts in the tissue of an aquatic snail. Note the prominent spines on the folded larva within the cyst, which are characteristic for the genus Paragordius; G) a Chordodes like larva on the outside gut wall of an aquatic beetle larva in the process of being melanized (orange-brown pigment). Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Once paratenic hosts ingest larvae, the larvae penetrate the gut and begin forming cysts within the tissue of their paratenic host. During cyst formation, larvae empty the contents of their pseudointestine. Laboratory studies by Dor- ier (1930), Poinar and Doelman (1974), Hanelt and Janovy (2003), Hanelt and colleagues (2012), and Bolek and colleagues (2010; 2013a; 2013b) report that during cyst formation, larvae secrete a jelly-like material from the pseudointes- tine and a clear halo-like structure appears around the folded larva (Figure 9). Transmission electron microscopy studies of gordiid cysts in tadpole paratenic hosts indicate that the clear halo-like cyst wall is multilayered (Poinar, 2010). Cyst development can take a few days up to a few months (Hanelt and Janovy, 2002; De Villalobos and Ronderos, 2003). More importantly, if an animal other than a definitive host ingests a paratenic host, the cysts are digested out and the larvae re-penetrate into the second paratenic hosts and re-encyst (De Villalobos and Ronderos, 2003; Hanelt and Janovy, 2003).
It is unknown if nematomorph larvae have any impact on mortality of paratenic hosts in nature. However, several laboratory studies and field observations indicate that insect paratenic hosts mount some type of immune reaction to horsehair worm larvae and cysts (Poinar and Doelman, 1974; De Villalobos and Ronderos, 2003; Hanelt and Janovy, 2003). Host reactions usually involve humoral mediated melanization of larvae and/or cysts (Figure 9). Melanization of gordiid larvae and cysts have been reported in a variety of aquatic larval insects including mosquitoes, chironomids, caddis- flies, mayflies, stoneflies, as well as larval beetles (Poinar and Doelman, 1974; Poinar, 1991; Hanelt and Janovy, 2003; Bolek et al., 2015).
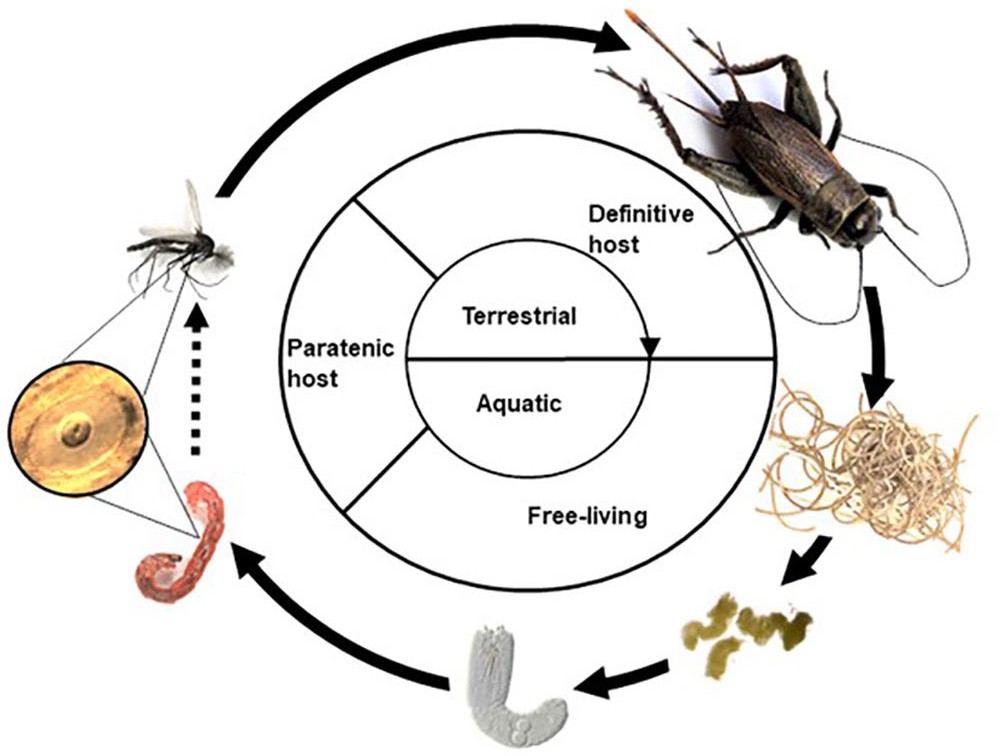
Figure 10. Diagram of a typical gordiid life cycle. The life cycle takes 4–8 weeks to complete in the laboratory depending on the species of nematomorph involved. Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Most definitive hosts for gordiids are predaceous or omnivorous arthropods, which capture infected paratenic arthropod hosts after they metamorphose from an aquatic habitat or scavenge on dead infected paratenic hosts (Figures 2, 10, and 11). Laboratory studies indicate that the maturation of gordiids within the definitive host takes several months. For example, the development within the definitive arthropod hosts can take as long as 8 months for Gordius tolosanus (Svábeník, 1925), 2 to 3 months for Chordodes japonensis, C. kenyaensis, and Paragordius obamai (Inoue, 1962; Hanelt et al., 2012; Bolek et al., 2013a), to as short as 1 month for P. varius (Hanelt and Janovy, 2004b).
Field studies indicate that after worms emerge from their hosts, only the gut remains within the host’s body cavity (Lin- stow, 1891; Thorne, 1940), whereas, other studies indicate that the production of eggs by female definitive hosts is inhibited or absent altogether (Tanner, 1939; Baker, 1985; Studier et al., 1991; Chiu et al., 2015). Only 1 report found that naturally infected female hosts might be capable of reproducing (Poulin, 1995). A more recent experimental study by Biron and colleagues (2005b) using naturally infected crickets showed that female crickets were capable of producing eggs only after they released worms and were provided with food ad libitum. However, all female crickets that released worms and produced eggs had difficulties mating with male crickets and/or ovipositing. In contrast, all infected male crickets were castrated by horsehair worms and did not regain the ability to produce sperm after they released worms.
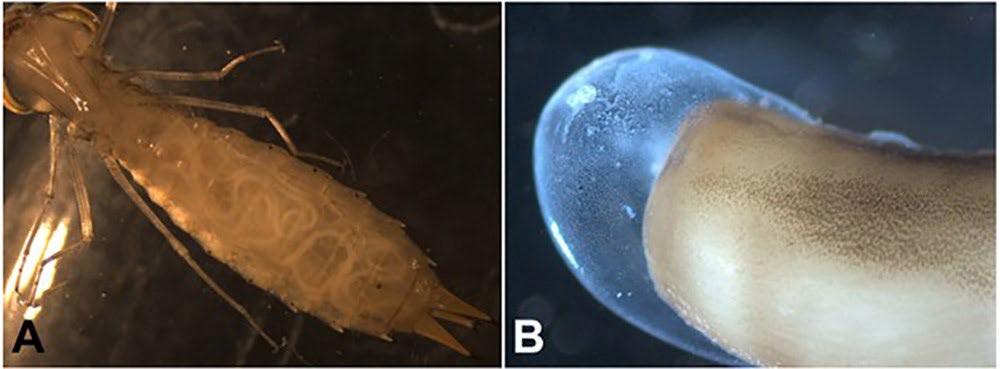
Figure 11. Development of gordiids in their arthropod definitive host. A) An infected larval comet darner Anax longipes, showing developing Neochordodes species within the hemocoel; B) a developing worm removed from the hemocoel of an arthropod host showing the thin larval cuticle. Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Life Cycle
One fascinating aspect of gordiid biology is their complex life cycle which includes both free-living and parasitic phases (May, 1919; Inoue, 1962; Hanelt and Janovy, 1999; 2004b; Hanelt et al., 2012; Bolek et al., 2013b; Swanteson-Franz et al., 2018) (Figure 10). As juveniles, gordiids are parasites of terrestrial arthropod hosts from which free-living adults emerge into aquatic or semi-aquatic environments, such as waterlogged fields, streams, rivers, and lakes (Hanelt et al., 2005; Anaya et al., 2019). Three species of gordiids (Paragordius varius, P. obamai, and Chordodes kenyaensis) have been domesticated in the laboratory including dioecious and parthenogenetic species (Hanelt and Janovy, 2004b; Hanelt et al., 2012; Bolek et al., 2013a; 2013b). Studies on these domesticated nematomorphs indicate that life cycles of gordiids involve 5 distinct life stages (Figure 10) including: 1) Egg strings, 2) free-living larvae, 3) parasitic cysts, 4) parasitic juveniles, and 5) dioecious or parthenogenetic free-living adults (Hanelt and Janovy, 2004a; 2004b; Hanelt et al., 2012). Juvenile gordiids are obligate parasites of predominately terrestrial arthropods, whereas a number of species of aquatic animals serve as paratenic hosts for the cyst stage (Hanelt et al., 2001; Bolek and Coggins, 2002; Hanelt and Janovy, 2003; 2004a).
As noted above in general, gordiids commonly infect 4 major groups of terrestrial arthropods, including beetles, or- thopterans, praying mantids, and cockroaches. Additional confirmed records exist from earwigs (order Dermaptera) and aquatic larval trichopterans and larval dragonflies (Schmidt- Rhaesa, 2013). All gordiids develop in the hemocoel of their arthropod host where they grow from a small length of 60– 100 μm to a length of over 2 m for some species (Schmidt- Rhaesa, 2013) (Figure 11). During development in the arthropod definitive host, 2 cuticles are present, a thin white larval cuticle which is replaced by a robust dark adult cuticle (Figure 7) (Schmidt-Rhaesa, 2005). Before emergence from their host, adult gordiids form an open wound on the posterior end of the host’s abdomen and once the infected arthropod enters water, the worms emerge head-first (Hanelt and Janovy, 2004b; Hanelt et al., 2012; Bolek et al., 2013a).
As noted, field observations indicate that infected terrestrial arthropods, such as crickets and beetles, deliberately en- ter water, suggesting that worms may be manipulating the behavior of their arthropod hosts (McCook, 1885; Müller, 1926; Jolivet, 1945; 1948). More recently, Thomas and colleagues (2003) discovered differences in the brains and concentrations of neurotransmitters among infected and uninfected field-collected crickets. Additional studies by Biron and colleagues (2005a; 2005b; 2006) show that several brain proteins are altered in crickets infected with gordiids. It is not known whether the gordiids’ mere presence or something emitted by the gordiids affects the hosts’ behavior.
Distribution and Diversity
Within the freshwater/terrestrial subphylum Gordiida, approximately 360 species of horsehair worms have been de- scribed worldwide from 18 extant and 2 extinct genera (Poinar, 1999; Poinar and Buckley, 2006; Yadav et al., 2018). In addition, 5 species of marine horsehair worms have been described from a single genus (Schmidt-Rhaesa, 2013). However, current estimates suggest that only 18% of the horsehair worm diversity has been documented across the world, with another 2,000 species awaiting discovery (Poinar, 2008). The earliest reported and credible fossil nematomorph, was described from 100 million year-old Lower Cretaceous Burmese amber and belongs to the extinct species Cretachordodes burmitis (Poinar and Buckley, 2006). Additionally, 2 individuals of the fossilized species Paleochordodes protus (Poinar, 1999) emerging from a cock- roach have been described from Dominican amber dated between 15 and 45 million years-old (Figure 12). However, obtaining knowledge on the diversity of horsehair worms has been difficult due to their unusual life cycles, where free-living adult worms exit their hosts, and the lack of reliable ways of collecting the free-living adults over large geographic areas from aquatic and terrestrial habitats (Bolek and Coggins, 2002; Bolek et al., 2013a; 2015).
The freshwater and terrestrial horsehair worms have been reported from all continents except Antarctica; whereas the marine genus Nectonema is known from several locations worldwide including both coasts of the northern Atlantic Ocean, the Indian Ocean, and the Pacific Ocean from the southern coast of New Zealand (Poinar and Brockerhoff, 2001; Hanelt et al., 2005; Bolek et al., 2015). The fauna of North American, European, and Argentinean nematomorphs has been relatively well studied. However, nematomorph diversity in Africa, Asia, and most of South America has received comparatively little attention (Hanelt et al., 2005; Schmidt-Rhaesa, 2013; Bolek et al., 2015; Schmidt-Rhaesa et al., 2016; Swanteson-Franz et al., 2018; Anaya et al., 2019; Zanca et al., 2020). Among the Nearctic freshwater and terrestrial horsehair worms, 24 species from 7 genera have been described (Schmidt-Rhaesa et al., 2003; Poinar and Chandler, 2004; Begay et al., 2012; Swanteson-Franz et al., 2018; Anaya et al., 2019). However, evidence from molecular barcod- ing techniques indicates there are numerous hidden, cryptic species. For example, the common Nearctic species Gordius robustus represents a large species complex composed of at least 8 distinct genetic lineages (Hanelt et al., 2015). These recent molecular studies indicate the importance of genetic data and limitations of morphological characters in determining some gordiid species within the phylum.
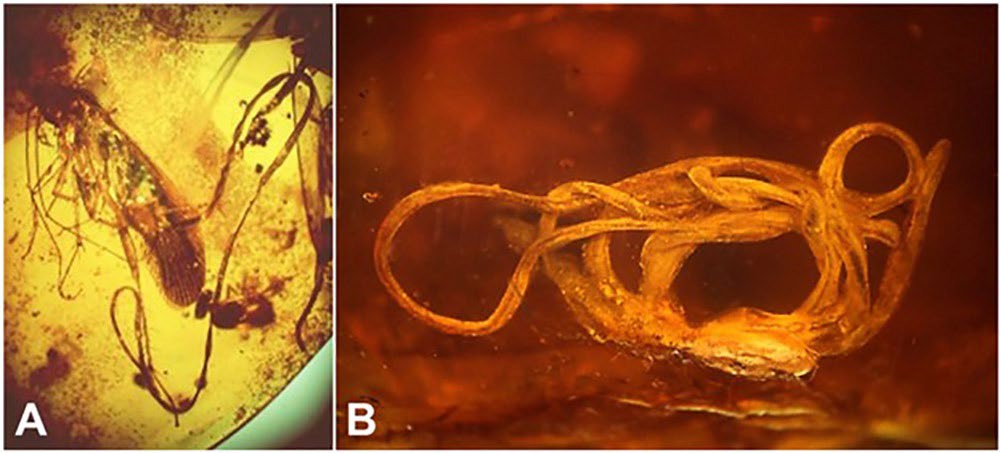
Figure 12. Fossil nematomorphs. A) Two specimens of Paleochordodes protus in the process of emerging from a cockroach host in Dominican amber, 15–45 Ma (= million years old); B) the oldest known hairworm fossil Cretachordodes burmitis recovered from Early Cretaceous amber, 100 Ma, from Myanmar. Source: G. Poinar. License: CC BY-NC-SA 4.0.
Taxonomy and Phylogeny
The phylum Nematomorpha consists of 2 subphyla, the marine Nectonematida and the freshwater and terrestrial Gordiida comprising 1, and 18 extant and 2 extinct genera, respectively (Schmidt-Rhaesa, 2013). Nematomorphs have few morphological characters that can be used by taxonomists for species determination. Macroscopic characters include the shape of the posterior end being bi-lobed or round in males, and tri-lobed or round in females. In addition, the presence of cuticular structures such as crescents near the cloacal opening and/or areas of dense bristles and/or spines on the posterior region of worms are useful for some genera delimitations (Figure 3) (Schmidt-Rhaesa, 2013). All other characters, including areoles and intra-areole spaces are found on the cuticle, many of which are so small that scanning electron microscopy (SEM) is necessary to visualize these characters. As such, SEM has become the standard protocol for nematomorph identification (Hanelt et al., 2005; Bolek et al., 2015).
Horsehair worms are placed in the superphylum Ecdysozoa and are considered the sister phylum to the phylum Nemata (Hanelt et al., 2005). However, and unlike nematodes, cephalic papillae, lateral epidermal cords, secretory-excretory systems, amphids, and spicules are lacking in horsehair worms. Other differences between horsehair worms and nematodes include genital openings located on the posterior end of female horsehair worms instead of near the middle of the body as in nematodes. Additionally, and unlike nematodes, these animals have a true larval stage that undergoes drastic morphological tissue reorganization during development in their host (Schmidt-Rhaesa, 1997; 2013).
Of 3 phylogenic hypotheses based on molecular (DNA) sequencing discussed here, the ancestor-descendant relationships of multiple genera and species within the phylum Nematomorpha were analyzed (Bleidorn et al., 2002; Chiu et al., 2017; Tobias et al., 2017). Bleidorn and colleagues (2002) use a combination of morphological and molecular (18S rRNA gene) data indicating a sister-group relationship between the marine genus Nectonema and the freshwater gordiids. However, within the subphylum Gordiida, all species within the basal genus Gordius and all species within the sister genus Paragordius are monophyletic. The remaining derived gen- era are not well supported and some appear as polyphyletic. For example, the more derived Neochordodes occidentalis is nested within species of Chordodes. More recent molecular phylogenetic analyses using mitochondrial markers (CO1) and/or nuclear markers (8S rRNA) indicate that the freshwater genus Paragordius is basal to the remaining freshwater and terrestrial gordiids. More importantly, these molecular phylogenetic hypotheses are in agreement with the traditional mor- phological relationships of freshwater and terrestrial gordiids including the genera Gordius and Acutogordius within the family Gordiidae and the remaining genera within the family Chordodidae (Chiu et al., 2017; Tobias et al., 2017).
Ecology and Behavior
The ecology of nematomorphs is closely tied to the biology of their arthropod definitive hosts and the aquatic or ter- restrial habitats of the adult free-living worms. However, few studies have sampled for free-living adult worms throughout the year and even fewer studies have examined multiple arthropod species for nematomorph infections (Bolek and Coggins, 2002; Poinar and Weissman, 2004; Looney et al., 2012). As a general rule and depending on the gordiid species, nematomorphs vary in their definitive host range, and free-living adults are seasonal and have a male-biased sex ratio (Hanelt et al., 2005; Bolek et al., 2015).
Host range for most nematomorph species is poorly understood and most host records are based on field observa- tions (Schmidt-Rhaesa, 1997; 2013; Schmidt-Rhaesa et al., 2003). Field studies indicate that some, but not all, nemato- morph species appear to be host-specific at the definitive arthropod host level (Poinar, 1991; Bolek and Coggins, 2002; Schmidt-Rhaesa et al., 2003; Chiu et al., 2011; Looney et al., 2012). For example, the North American Chordodes mor- gani has been reported from 4 phylogenetically distinct orthopteran and cockroach species, suggesting that some horsehair worms are generalists at the definitive host level (Schmidt-Rhaesa et al., 2003). However, other species, particularly in the Gordius cf. robustus complex, appear to be more specific at the definitive host level and are restricted to a single or a few closely related species of arthropod hosts (Hanelt et al., 2015). Molecular evidence from mitochondrial (CO1 and cytB) and nuclear (partial 28S, ITS1, 5.8S, and ITS2) DNA suggests that at least 8 species occur across North America. However, this group is paraphyletic, since the European G. aquaticus and G. balticus group among the G. robustus lineages form 2 distinct clades, A and B (Figure 13B). When all known arthropod definitive hosts are mapped onto this phylogeny it appears that species within clade A infect various species of orthopterans; whereas species in clade B infect millipedes and ground beetles (Figure 13B).
Once emerged from their arthropod hosts, free-living adult worms are seasonal (Bolek and Coggins, 2002; Schmidt-Rhaesa et al., 2005; Salas et al., 2011; Anaya, 2019). For example, Bolek and Coggins (2002) reported the occurrence of free-living adults of Gordius difficilis in Wisconsin, United States from June to October; whereas Anaya (2019) reported G. terrestris (incidentally, the only known species of gordiid consistently collected from terrestrial habitats) from Oklahoma, United States during October through March. Additionally, Salas and colleagues (2011) examined the seasonal occurrence of free-living individuals of 4 species of sympatric gordiids over a 1-year period from a stream in Argentina. In their study, free-living worms of all 4 species occurred in the stream during the fall, winter, and spring. However, Noteochorododes cymatium, N. talensis, and Pseudochordodes dugesi were most abundant during the winter and spring; whereas Chordodes brasiliensis was most abundant during the fall.
The explanation for these seasonal patterns includes the short life span of free-living adult worms (2–8 weeks) and the abundance of their arthropod definitive hosts. For example, in a 3-year study, Schmidt-Rhaesa and colleagues (2005) collected data on recently emerged adults of 2 species of nematomorphs and their definitive arthropod hosts around a swimming pool in southern France. Most adults of Pseudochordodes tricuspidatus emerged from their hosts during June through August, whereas most adults of Spinochordodes tellinii emerged from their hosts during August through September. At their study site, both gordiid species infected different species of definitive hosts and their occurrence was correlated with the abundance of these hosts.
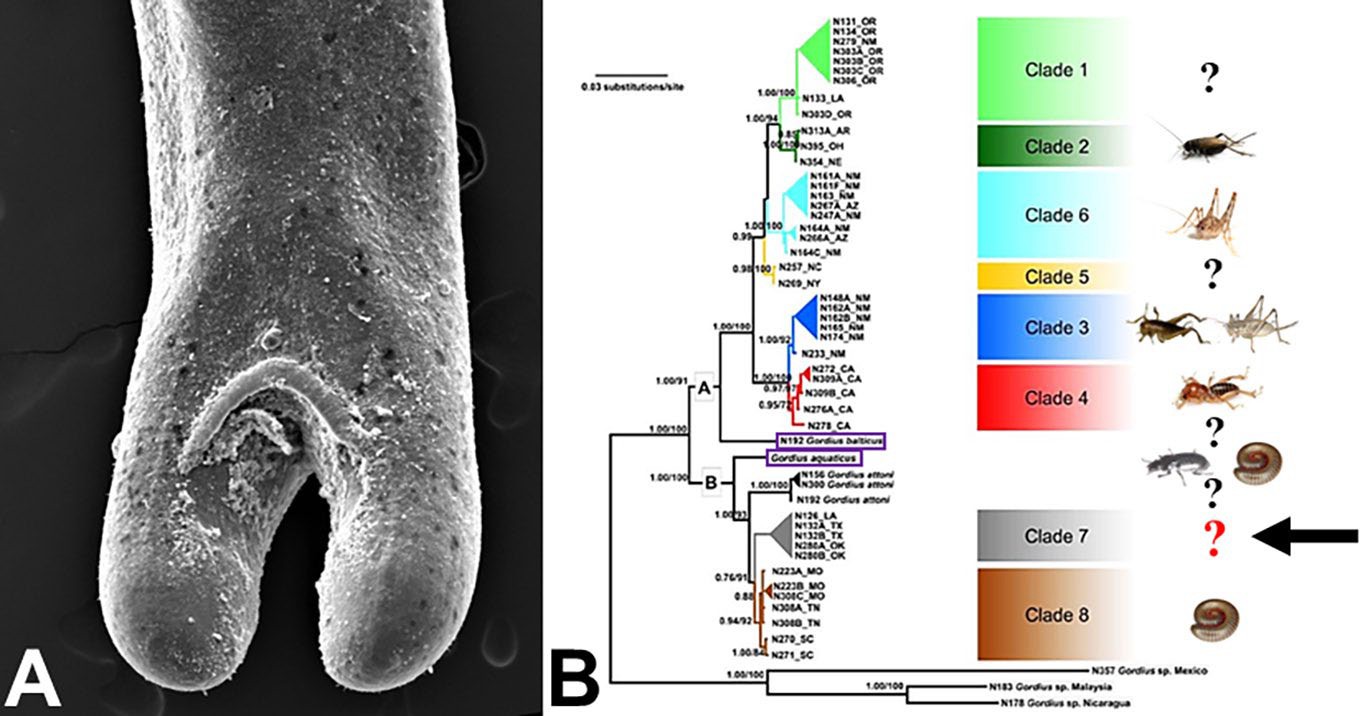
Figure 13. Hosts and phylogenetic relationships of the Gordius cf. robustus complex. A) Posterior end of a male G. cf. robustus, with the characteristic bi-lobed end, post cloacal crescent, and poorly developed areoles on the cuticle; B) phylogenetic hypothesis (partial CO1 and cytB sequences) of the G. cf. robustus group and diverse group of arthropod definitive hosts. Note that the G. cf. robustus lineages are paraphyletic with the European species (purple brackets) group among the G. robustus lineages and form 2 distinct clades, A and B. Species in clade A appear to infect orthopteran arthropod hosts, whereas, species in clade B infect ground beetle and millipede arthropod hosts. Sources: A) M. G. Bolek; B) adapted from Hanelt et al., 2015. License: CC BY-NC-SA 4.0.
Free-living adults do not feed and are found in various aquatic habitats including water sources in caves, puddles, ponds, lakes, and small and large streams and rivers (Reeves, 2000; Hanelt et al., 2005; Schmidt-Rhaesa, 2013; Bolek et al., 2015). Within these habitats, free-living worms can be located in the sediment, among moist fallen leaves, under rocks, in algal mats, and/or on aquatic vegetation where they form Gordian knots and mate (Hanelt et al., 2005; Bolek et al., 2015). Additionally, free-living adults of Gordius terrestris, a terrestrial species, appear during rain events on wet lawns and pools of water on streets and sidewalks, where the worms copulate. After the rains stop, adult free-living worms can be found entangled in the roots of grasses and in the soil where females deposit egg strings (Anaya, 2019; Anaya et al., 2019; Figure 14).
The sex ratio of free-living adult gordiids is usually but not always male biased, with a few field studies indicating equal sex ratios (De Villalobos and Camino, 1999; Valvassori et al., 1988; Salas et al., 2011). For example, Cochran and colleagues (1999) reported that of 1,391 individuals of Gordius difficilis collected during a 32-year period in 6 Midwestern states of the United States, 1,205 were males. In contrast, Watermolen and Haen (1994) reported 67 individuals of G. robustus from Wisconsin of which 66 were females. These field-skewed sex ratios are in contrast to laboratory life cycle studies on dioecious nematomorph species. Hanelt and Janovy (2004b) and Bolek and colleagues (2013b) each found no statistically significant differences in the sex ratios of Paragordius varius or Chordodes kenyaensis emerging from laboratory-reared and -infected cricket definitive hosts. A few hypotheses have been proposed for this strongly skewed sex ratio in the field, including differences in the development times of male and female worms in their final hosts, and/or behavior differences among free-living males and females (Poulin, 1996; Bolek and Coggins, 2002). More recently, Anaya (2019) documented behavioral differences among male and female Gordius terrestris, a species with an extremely male biased sex ratio (5.4:1.0) observed in the field. In the laboratory, when male and female worms are placed on the surface of the soil, significantly more females burrow into the soil than males. Once females burrow, they begin ovipositing. This observation is important and provides a plausible explanation for the extremely male-biased sex ratio observed for G. terrestris in the field. Taken together, these observations suggest that unlike males, after mating female horsehair worms may be moving to specific locations in the environment to oviposit and be more difficult to locate than males.

Figure 14. Typical habitat for free-living adults, larvae, and cysts of freshwater and terrestrial gordiids. A) Second-order stream in Payne County, Oklahoma, United States; B) a typical first-order stream in the Chiricahua Mountains, Arizona, United States; C) a white free-living adult male Gordius sp. on the bottom of a first-order stream in the Chiricahua Mountains, Arizona, United States; D) a third-order stream in the Córdoba, Argentina. Many of the nematomorphs glue their egg strings on rocks in this habitat; E) adult free-living G. terrestris entangled in grass roots under the soil, in a suburban environment, Stillwater, Oklahoma, United States. Source: M. G. Bolek. License: CC BY-NC-SA 4.0.
Little information is available on the physiological constraints of free-living stages of horsehair worms to their exter- nal environment. However, Bolek and colleagues (2013b) indicate that in laboratory cultures adult free-living Chordodes kenyaensis worms die within 24 hours if they emerge from their hosts in cages without a water source, suggesting that adult free-living worms must remain moist to survive. In addition, Achiorno and colleagues (2008) examined the survival of eggs, larvae, and free-living adults of C. nobilii to extreme temperatures. They demonstrated that all eggs, most larvae, and all adult gordiids die at a high temperature of 40.5 °C, and all eggs and most adult worms (89%) die at a low temperature of −3 °C. In contrast, larvae frozen at −3 °C for 48 hours survived freezing and are capable of infecting mosquito larva paratenic hosts. Finally, Bolek and colleagues (2013a) evaluated the survival of larvae and cysts of North American and African gordiids in the genus Paragordius when super cooled and/or frozen at −20 °C or −80 °C for up to 7 months. Their work demonstrates that post-frozen larvae and cysts of these species have the ability to infect and develop in the next host in the life cycle. It is currently unclear why larvae and cysts of gordiid species from Africa have the ability to survive super cooling and/or freezing for such periods.
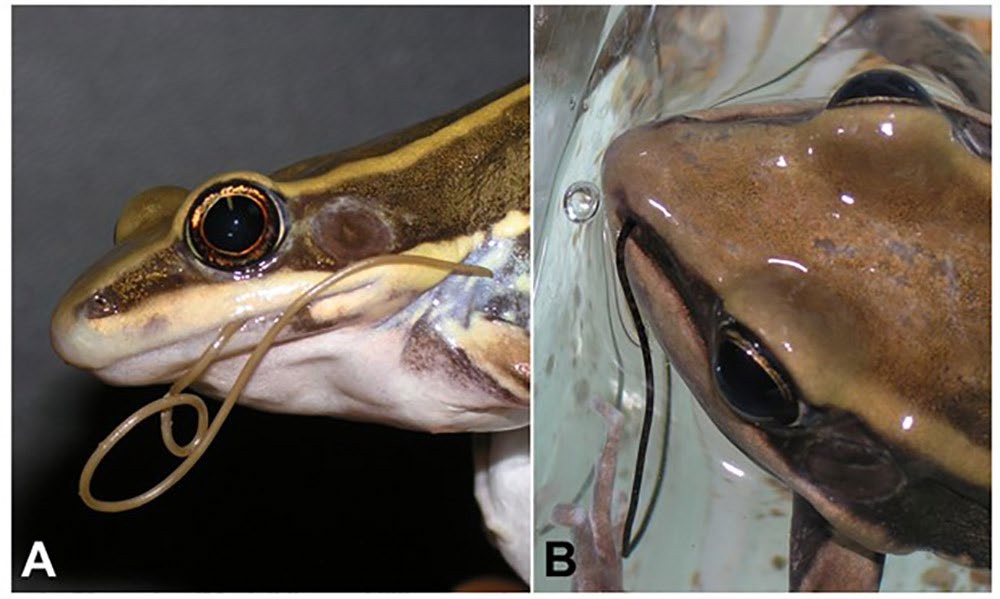
Figure 15. Predators and epibionts of gordiids. A, B) A free-living Paragordius tricuspidatus escaping from the mouth (A) and nose (B) of the common green frog Rana erythraea after it ingested an infected cricket. Source: F. Thomas. License: CC BY-NC-SA 4.0.
Finally, birds, fish, and frogs occasionally eat free-living adult nematomorphs and their infected hosts (Cochran et al., 1999; Bolek and Coggins, 2002; De Villalobos et al., 2008; Fair et al., 2010). However, work by Sato and colleagues (2008; 2011) in Japan indicates that gordiids are only found in the stomachs of trout (Salvelinus leucomaenis japonicus) when those fish also consume their camel cricket hosts. As a result, Sato and colleagues (2011) hypothesized that horsehair worm infections and their manipulations of terrestrial arthropod hosts can increase energy inputs into aquatic ecosystems. Using estimates of seasonal prey abundance, they argue that, during peak worm emergence times, infected orthopterans account for 60% of the annual energy intake of Japanese trout (Sato et al., 2011). Other studies by Ponton and colleagues (2006a; 2006b) indicate that when trout and frogs consume orthopterans infected with these nematomorphs, 18–35% of the ingested nematomorphs can escape the predators of their hosts, through the mouth or nose of frogs and the mouth or gills of fish (Figure 15).
Original Chapter 57 Authors:
Matthew G. Bolek
Department of Integrative Biology, Oklahoma State University, Stillwater, Oklahoma, United States bolek@okstate.edu
Ben Hanelt
Department of Biology, University of New Mexico, Albuquerque, New Mexico, United States bhanelt@unm.edu
Revisions: Reordered the chapter to match new course organization.
Literature Cited
Achiorno, C. L., L. Ferrari, and C. De Villalobos. 2008. Effect of extreme temperature on egg development, larval and adult survival of Chordodes nobilii Camerano, 1901 (Gordiida, Nematomorpha). Acta Parasitologica 53: 392–396. doi: 10.2478/s11686-008-0052-5
Anaya, C. 2019. Comparative study of life cycle ecology and host-parasite interactions of horsehair worms (Phylum: Nematomorpha). PhD thesis, Oklahoma State University, Stillwater, Oklahoma, United States.
Anaya, C., A. Schmidt-Rhaesa, B. Hanelt, and M. G. Bolek. 2019. A new species of Gordius (Phylum Nematomorpha) from terrestrial habitats in North America. ZooKeys 892: 59–75. doi: 10.3897/zookeys.892.38868
Baker, G. H. 1985. Parasites of the millipede Ommatoiulus moreletii (Lucas) (Diplopoda: Iulidae) in Portugal, and their potential as biological control agents in Australia. Australian Journal of Zoology 33: 23–32. doi: 10.1071/ZO9850023
Begay, A. C., A. Schmidt-Rhaesa, M. G. Bolek, and B. Hanelt. 2012. Two new Gordionus species (Nematomorpha: Gordiida) from the southern Rocky Mountains (USA). Zootaxa 3406: 30–38. doi: 10.11646/zootaxa.3406.1.2
Biron, D. G., L. Marché, F. Ponton, H. D. Loxdale, et al. 2005a. Behavioural manipulation in a grasshopper harboring hairworms: A proteomics approach. Proceedings of the Royal Society B 272: 2,117–2,126. doi: 10.1098/ rspb.2005.3213
Biron, D. G., F. Ponton, C. Joly, A. Menigoz, et al. 2005b. Water-seeking behavior in insects harboring hairworms: Should the host collaborate? Behavioral Ecology 16: 656–660. doi: 10.1093/beheco/ari039
Biron, D. G., F. Ponton, L. Marché, N. Galeotti, et al. 2006. ‘Suicide’ of crickets harboring hairworms: A proteomics investigation. Insect Molecular Biology 15: 731–742. doi: 10.1111/j.1365-2583.2006.00671.x
Bleidorn, C., A. Schmidt-Rhaesa, and J. R. Garey. 2002. Systematic relationships of Nematomorpha based on molecular and morphological data. Invertebrate Biology 121: 357–364. doi: 10.1111/j.1744-7410.2002.tb00136.x
Bolek, M. G. 2000. Records of horsehair worms Paragordius varius, Chordodes morgani and Gordius robustus (Nematomorpha) from Indiana. Journal of Freshwater Ecology 15: 421–423. doi: 10.1080/02705060.2000.9663760
Bolek, M. G., and J. R. Coggins. 2002. Seasonal occurrence, morphology, and observations on the life history of Gordius difficilis (Nematomorpha: Gordioidea) from southeastern Wisconsin, United States. Journal of Parasitology 88: 287–294. doi:10.1645/0022-3395(2002)088[0287:SOMAOO]2.0.CO;2
Bolek, M. G., E. Rogers, C. Szmygiel, R. P. Shannon, et al. 2013a. Survival of larval and cyst stages of gordiids (Nematomorpha) after exposure to freezing. Journal of Parasitology 99: 397–402. doi: 10.1645/12-62.1
Bolek, M. G., A. Schmidt-Rhaesa, C. L. De Villalobos, and B. Hanelt. 2015. Phylum Nematomorpha. In J. Thorp and D. C. Rogers, eds. Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates, Volume 1, 4th edition. Academic Press, Cambridge, Massachusetts, United States, p. 303–326. doi: 10.1016/B978-0-12-385026-3.00015-2
Bolek, M. G., A. Schmidt-Rhaesa, B. Hanelt, and D. J. Richardson. 2010. Redescription of the African Chordodes albibarbatus Montgomery 1898, and description of Chordodes janovyi n. sp. (Gordiida, Nematomorpha) and its non-adult stages from Cameroon, Africa. Zootaxa 2631: 36–54. doi: 10.11646/zootaxa.2631.1.3
Bolek, M. G., C. Szmygiel, A. Kubat, A. Schmidt-Rhaesa, et al. 2013b. Novel techniques for biodiversity studies of gordiids and description of a new species of Chordodes (Gordiida, Nematomorpha) from Kenya, Africa. Zootaxa 3717: 23–38. doi: 10.11646/zootaxa.3717.1.2
Brivio, M. F., M. De Eguileor, A. Grimaldi, D. Vigetti, et al. 2000. Structural and biochemical analysis of the parasite Gordius villoti (Nematomorpha, Gordiacea) cuticle. Tissue and Cell 32: 366–376. doi: 10.1054/tice.2000.0125
Chiu, M.-C., C.-G. Huang, W.-J. Wu, and S.-F. Shiao. 2016. Annual survey of horsehair worm cysts in northern Taiwan, with notes on a single seasonal infection peak in chironomid larvae (Diptera: Chironomidae). Journal of Parasitology 102: 319–326. doi: 10.1645/15-907
Chiu, M.-C., C.-G. Huang, W.-J. Wu, and S.-F. Shiao. 2015. Morphological allometry and intersexuality in horsehair- worm-infected mantids, Hierodula formosana (Mantodea: Mantidae). Parasitology 142: 1,130–1,142. doi: 10.1017/ S0031182015000360
Chiu, M.-C., C.-G. Huang, W.-J. Wu, and S.-F. Shiao. 2011. A new horsehair worm, Chordodes formosanus sp. n. (Nematomorpha, Gordiida) from Hierodula mantids of Taiwan and Japan with redescription of a closely related species, Chordodes japonensis. ZooKeys 160: 1–22. doi: 10.3897/zookeys.160.2290
Chiu, M.-C., C.-G. Huang, W.-J. Wu, and S.-F. Shiao.2017. A new orthopteran-parasitizing horsehair worm, Acutogordius taiwanensis sp. n., with a redescription of Chordodes formosanus and novel host records from Taiwan (Nematomorpha, Gordiida). ZooKeys 683: 1–23. doi: 10.3897/zookeys.683.12673
Cochran, P. A., A. P. Kinziger, and W. J. Poly. 1999. Predation on horsehair worms (Phylum Nematomorpha). Journal of Freshwater Ecology 14: 211–218. doi: 10.1080/02705060.1999.9663672
De Villalobos, L. C., and N. Camino. 1999. Two new species of Gordiacea (Nematomorpha) parasites of Stagmatoptera hyaloptera (Mantidae) from Argentina. Iheringia Série Zoologia 86: 71–76.
De Villalobos, L. C., and M. Ronderos. 2003. Dasyhelea necrophila Spinelli et Rodriguez, 1999 (Diptera, Ceratopogonidae) a new potential paratenic host of Paragordius varius (Leidy, 1851) (Gordiida, Nematomorpha). Acta Parasitologica 48: 218–221.
De Villalobos, L. C., J. J. Ortiz-Sandoval, and E. Habit. 2008. Finding of Gordius austrinus de Villalobos, Zanca and Ibarra-Vidal, 2005 (Gordiida, Nematomorpha) in the stomach of Salmo trutta (Salmoniformes) in Patagonia. Gayana 72: 31–35.
Dorier, A. 1930. Classe des Gordiaces. In P.-P. Grassé, ed. Traite de zoologie, Volume 4. Masson, Paris, France, p. 1,201– 1,222.
Fair, J. M., B. Hanelt, and K. Burnett. 2010. Horsehair worms (Gordius robustus) in nests of the western bluebird (Sialia mexicana): Evidence for antipredator avoidance? Journal of Parasitology 96: 429–430. doi: 10.1645/GE-2313.1
Hanelt, B. 2009. An anomaly against a current paradigm: Extremely low rates of individual fecundity variability of the Gordian worm (Nematomorpha: Gordiida). Parasitology 136: 211-218. doi: 10.1017/S0031182008005337
Hanelt, B., and J. J. Janovy, Jr. 2004a. Life cycle and paratenesis of American gordiids (Nematomorpha: Gordiida). Journal of Parasitology 90: 240–244. doi: 10.1645/GE-78R
Hanelt, B., and J. J. Janovy, Jr. 1999. The life cycle of a horsehair worm, Gordius robustus (Nematomorpha: Gordioidea). Journal of Parasitology 85: 139–141.
Hanelt, B., and J. J. Janovy, Jr. 2002. Morphometric analysis of nonadult characters of common species of American gordiids (Nematomorpha: Gordioidea). Journal of Parasitology 88: 557–562. doi: 10.1645/0022-3395(2002)088[0557:MAONCO]2.0.CO;2
Hanelt, B., and J. J. Janovy, Jr. 2003. Spanning the gap: Experimental determination of paratenic host specificity of horse- hair worms (Nematomorpha: Gordiida). Invertebrate Biology 122: 12–18. doi: 10.1111/j.1744-7410.2003.tb00068.x
Hanelt, B., and J. J. Janovy, Jr. 2004b. Untying the gordian knot: The domestication and laboratory maintenance of a gordian worm, Paragordius varius (Nematomorpha: Gordiida). Journal of Natural History 38: 939–950. doi: 10.1080/0022293021000058718
Hanelt, B., M. G. Bolek, and A. Schmidt-Rhaesa. 2012. Going solo: Discovery of the first parthenogenetic gordiid (Nematomorpha: Gordiida). PLoS One 7: e34472. doi: 10.1371/journal.pone.0034472
Hanelt, B., L. E. Grother, and J. J. Janovy, Jr. 2001. Physid snails as sentinels of freshwater nematomorphs. Journal of Parasitology 87: 1,049–1,053. doi: 10.1645/0022-3395(2001)087[1049:PSASOF]2.0.CO;2
Hanelt, B., A. Schmidt-Rhaesa, and M. G. Bolek. 2015. Cryptic species of hairworm parasites revealed by molecular data and crowdsourcing of specimen collections. Molecular Phylogenetics and Evolution 82: 211–218. doi: 10.1016/j. ympev.2014.09.010
Hanelt, B., F. Thomas, and A. Schmidt-Rhaesa. 2005. Biology of the phylum Nematomorpha. Advances in Parasitology 59: 243–305. doi: 10.1016/S0065-308X(05)59004-3
Harkins, C., R. Shannon, M. Papeş, A. Schmidt-Rhaesa, et al. 2016. Using gordiid cysts to discover the hidden diversity, potential distribution, and new species of gordiids (Phylum Nematomorpha). Zootaxa 4088: 515–530. doi: 10.11646/ zootaxa.4088.4.3
Inoue, I. 1960. Studies on the life history of Chordodes japonensis, a species of Gordiacea, II: On the manner of entry into aquatic insect larvae of Chordodes larvae. Annotationes Zoologicae Japonenses 33: 132–141.
Inoue, I. 1962. Studies on the life history of Chordodes japonensis, a species of Gordiacea, III: The modes of infection. Annotationes Zoologicae Japonenses 35: 12–19.
Jolivet, P. 1945. De l’hydrotrophisme positif de Steropus madidus, Fabr. (Col., Pterostichidae). Miscellanea Entomologica 41: 102–106.
Jolivet, P. 1948. Introduction a l’étude des Gordiacés, vers parasites d’insectes. Miscellanea Entomologica 45: 83–90.
Linstow, O. 1891. Weitere Beobachtungen an Gordius tolosanus und Mermis. Archiv für Mikroskopische Anatomie 37: 239–249. doi: 10.1007/BF02954296
Looney, C., B. Hanelt, and R. S. Zack. 2012. New records of nematomorph parasites (Nematomorpha: Gordiida) of ground beetles (Colepoptera: Carabidae) and camel crickets (Orthoptera: Rhaphidophoridae) in Washington State. Journal of Parasitology 98: 554–559. doi: 10.1645/GE- 2929.1
May, H. G. 1919. Contributions to the life histories of Gordius robustus Leidy and Paragordius varius (Leidy). Illinois Biological Monographs 5: 1–119.
McCook, H. C. 1885. Note on the intelligence of a cricket parasitized by a Gordius. Annals and Magazine of Natural History, Series 5, 15: 275–276.
Müller, G. W. 1926. Über Gordiaceen. Zeitschrift für die Morphologie und Ökologie der Tiere 7: 134–270. doi: 10.1007/BF00540721
Müller, M. C. M., R. Jochmann, and A. Schmidt-Rhaesa. 2004. The musculature of horsehair worm larvae (Gordius aquaticus, Paragordius varius, Nematomorpha): F-actin staining and reconstruction by cLSM and TEM. Zoomorphology 123: 45–54. doi: 10.1007/s00435-003-0088-x
Poinar, Jr., G. O. 2008. Global diversity of hairworms (Nematomorpha: Gordiaceae) in freshwater. Hydrobiologia 595: 79–83. doi: 10.1007/s10750-007-9112-3
Poinar, Jr., G. O. 1991. Nematoda and Nematomorpha. In J. H. Thorp and A. P. Covich, eds. Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego, California, United States, p. 249–283.
Poinar, Jr., G. O. 2010. Nematoda and Nematomoprha. In J. H. Thorp and A. P. Covich, eds. Ecology and Classification of North American Freshwater Invertebrates, 3rd edition. Academic Press, San Diego, California, United States, p. 237–276.
Poinar, Jr., G. O. 1999. Palaeochordodes protus n. g., n. sp. (Nematomorpha, Chordodidae), parasites of a fossil cockroach, with a critical examination of other fossil hairworms and helminths of extant cockroaches (Insecta: Blattaria). Invertebrate Biology 118: 109–115. doi: 10.2307/3227053
Poinar, Jr., G. O., and A. M. Brockerhoff. 2001. Nectonema zealandica n. sp. (Nematomorpha: Nectonematoidea) parasitizing the purple rock crab Hemigrapsus edwardsi (Brachyura: Decapoda) in New Zealand, with notes on the prevalence of infection and host defense reactions. Systematic Parasitology 50: 149–157. doi: 10.1023/A:1011961029290
Poinar, Jr., G. O., and R. Buckley. 2006. Nematode (Nematoda: Mermithidae) and hairworm (Nematomorpha: Chordodidae) parasites in early cretaceous amber. Journal of Invertebrate Pathology 93: 36–41. doi: 10.1016/j.jip.2006.04.006
Poinar, Jr., G. O., and C. M. Chandler. 2004. Synopsis and identification of North American hairworms (Gordioidea: Nematomorpha). Journal of the Tennessee Academy of Sciences 79: 1–7.
Poinar, Jr., G. O., and J. J. Doelman. 1974. A reexamination of Neochordodes occidentalis (Montg.) comb. n. (Chordodidae: Gordioidea): Larval penetration and defense reaction in Culex pipiens L. Journal of Parasitology 60: 327–335. doi: 10.2307/3278476
Poinar, Jr., G. O., and D. B. Weissman. 2004. Hairworm and nematode infections of North American Jerusalem crickets, field crickets, and katydids (Orthoptera; Stenopelmatidae, Gryllidae and Tettigonidae). Journal of Orthopteran Research 13: 143–147. doi: 10.1665/1082-6467(2004)013[0143:HANION]2.0.CO;2
Ponton, F., C. Lebarbenchon, T. Lefèvre, D. G. Biron, et al. 2006a. Parasite survives predation on its host. Nature 440: 756. doi: 10.1038/440756a
Ponton, F., C. Lebarbenchon, T. Lefèvre, F. Thomas, et al. 2006b. Hairworm anti-predator strategy: A study of causes and consequences. Parasitology 133: 631–638. doi: 10.1017/ S0031182006000904
Poulin, R. 1995. Hairworms (Nematomorpha: Gordioidea) infecting New Zealand short-horned grasshoppers (Orthoptera: Acrididae). Journal of Parasitology 81: 121–122. doi: 10.2307/3284023
Poulin, R. 1996. Observations on the free-living adult stage of Gordius dimorphus (Nematomorpha: Gordioidea). Journal of Parasitology 82: 845–846. doi: 10.2307/3283905
Protasioni, M., M. De Eguileor, T. Congiu, A. Grimaldi, et al. 2003. The extracellular matrix of the cuticle of Gordius panigettensis (Gordioiidae, Nematomorpha): Observations by TEM, SEM, and AFM. Tissue and Cell 35: 306–311. doi: 10.1016/s0040-8166(03)00052-1
Reeves, W. K. 2000. Invertebrate cavernicoles of the Great Smoky Mountains National Park, USA. Journal of the Elisha Mitchell Scientific Society 116: 334–343.
Restelli, M., C. L. De Villalobos, and F. Zanca. 2002. Ultrastructural description of the musculature, the intraepidermal nervous system and its basi-epidermal interrelation in Pseudochordodes bedriagae (Nematomorpha). Cell and Tissue Research 308: 299–306. doi: 10.1007/s00441-001-0487-6
Reutter, K. 1972. Gordius, das Wasserkalb. Mikrokosmos 61: 198–204. doi: 10.1007/s00441-001-0487-6
Salas, L., C. L. De Villalobos, and F. Zanca. 2011. Sexual size dimorphism, sex ratio, and the relationship between seasonality and water quality in four species of Gordiida (Nematomorpha) from Catamarca, Argentina. Journal of Helminthology 85: 319–324. doi: 10.1017/ S0022149X1000057X
Sato, T., M. Arizono, R. Sone, and Y. Harada. 2008. Parasite- mediated allochtonous input: Do hairworms enhance subsidized predation of stream salmonids on crickets? Canadian Journal of Zoology 86: 1–5. doi: 10.1139/Z07-135
Sato, T., K. Watanabe, M. Kanaiwa, Y. Niizuma, et al. 2011. Nematomorph parasites drive energy flow through a riparian ecosystem. Ecology 92: 201–207. doi: 10.1890/09-1565.1 Schmidt-Rhaesa, A. 2005. Morphogenesis of Paragordius varius (Nematomorpha) during the parasitic phase. Zoomorphology 124: 33–46. doi: 10.1007/s00435-005-0109-z
Schmidt-Rhaesa, A. 2013. Nematomorpha. In A. Schmidt-Rhaesa, ed. Handbook of Zoology: Gastrotricha, Cycloneuralia and Gnathifera, Nematomorpha, Priapulida, Kinorhyncha, and Loricifera, Volume 1. De Gruyter, Berlin, Germany, p. 29–145.
Schmidt-Rhaesa, A. 1997. Nematomorpha. In J. Schwoerbel and P. Zwick, eds. Süßwasserfauna Mitteleuropas. Fischer, Stuttgart, Germany, p. 1–124.
Schmidt-Rhaesa, A. 1996a. Ultrastructure of the anterior end in three ontogenetic stages of Nectonema munidae (Nematomorpha). Acta Zoologica 77: 267–278. doi: 10.1111/ j.1463-6395.1996.tb01271.x
Schmidt-Rhaesa, A. 1996b. Zur Morphologie, Biologie und Phylogenie der Nematomorpha: Untersuchungen an Nectonema munidae und Gordius aquaticus. Cuvillier Verlag, Göttingen, Germany, 276 p.
Schmidt-Rhaesa, A., D. G. Biron, C. Joly, and F. Thomas. 2005. Host-parasite relations and seasonal occurrence of Paragordius tricuspidatus and Spinochordodes tellinii (Nematomorpha) in Southern France. Zoologischer Anzeiger 244: 51–57. doi: 10.1016/j.jcz.2005.04.002
Schmidt-Rhaesa, A., C. De Villalobos, F. Zanka, B. Hanelt, et al. 2016. Phylum Nematomorpha. In J. Thorp and D. C. Rogers, eds. Keys to Nearctic Fauna: Freshwater Invertebrates, Volume 2, 4th edition. Academic Press, Cambridge, Massachusetts, United States, p. 181–188.
Schmidt-Rhaesa, A., B. Hanelt, and W. K. Reeves. 2003. Redescription and compilation of Nearctic freshwater Nematomorpha (Gordiida), with the description of two new species. Proceedings of the Academy of Natural Sciences of Philadelphia 153: 77–117. doi: 10.1635/0097-3157(2003)153[0077:RACONF]2.0.CO;2
Singh, S. N., and V. G. Rao. 1966. On a case of human infection with a gordiid worm in the orbit. Indian Journal of Helminthology 18: 65–67.
Studier, E. H., K. H. Lavoit, and C. M. Chandler. 1991. Biology of cave crickets, Hadenoecus subterraneus, and camel crickets, Ceuthophilus stygius (Insecta: Orthoptera): Parasitism by hairworms (Nematomorpha). Journal of the Helminthological Society of Washington 58: 248–250. https://archive.org/details/journal-helminthological-society- washington-58-002-248-250
Švábeník, J. 1925. [Parasitism and metamorphosis of the species Gordius tolosanus Duj. (Parasitismus a metamorfosa druhu Gordius tolosanus Duj.).] Publications of the Faculty of Science of Masaryk University 58: 1–48. [In Czech with English summary.]
Swanteson-Franz, R. J., D. A. Marquez, C. I. Goldstein, A. Schmidt-Rhaesa, et al. 2018. New hairworm (Nematomorpha, Gordiida) species described from the Arizona Madrean Sky Islands. ZooKeys 733: 131–145. doi: 10.3897/zookeys.733.22798
Szmygiel, C., A. Schmidt-Rhaesa, B. Hanelt, and M. G. Bolek. 2014. Comparative descriptions of non-adult stages of four genera of Gordiids (Phylum: Nematomorpha). Zootaxa 3768: 101–118. doi: 10.11646/zootaxa.3768.2.1
Tanner, V. M. 1939. Notes on the Gordiacea of Utah. Great Basin Naturalist 1: 2.
Thomas, F., A. Schmidt-Rhaesa, G. Martin, C. Manu, et al. 2002. Do hairworms (Nematomorpha) manipulate the water-seeking behavior of their terrestrial hosts? Journal of Evolutionary Biology 15: 356–361. doi: 10.1046/j.1420- 9101.2002.00410.x
Thomas, F., P. Ulitsky, R. Augier, N. Dusticier, et al. 2003. Biochemical and histological changes in the brain of the cricket Nemobius sylvestris infected by the manipulative parasite Paragordius tricuspidatus (Nematomorpha). International Journal for Parasitology 33: 435–443. doi: 10.1016/s0020-7519(03)00014-6
Thorne, G. 1940. The hairworm, Gordius robustus Leidy, as a parasite of the Mormon cricket, Anabrus simplex Haldeman. Journal of the Washington Academy of Sciences 30: 219– 231.
Tobias, Z. J. C., A. K. Yadav, A. Schmidt-Rhaesa, and R. Poulin. 2017. Intra- and interspecific genetic diversity of New Zealand hairworms (Nematomorpha). Parasitology 144: 1,026–1,040. doi: 10.1017/S0031182017000233
Valvassori, R., G. Scarì, M. De Eguileor, L. D. Lernia, et al. 1988. Gordius villoti (Nematomorpha) life cycle in relation with caddis fly larvae. Bolletino di zoologia 55: 269–278.
Watermolen, D. J., and G. L. Haen. 1994. Horsehair worms (phylum Nematomorpha) in Wisconsin, with notes on their occurrence in the Great Lakes. Journal of Freshwater Ecology 9: 7–11. doi: 10.1080/02705060.1994.9664421
Yadav A. K., Z. J. C. Tobias, and A. Schmidt-Rhasesa. 2018. Gordionus maori (Nematomorpha: Gordiida), a new species of horsehair worm from New Zealand. New Zealand Journal of Zoology 45: 29–42. doi: 10.1080/03014223.2017.1329155
Yamashita, J., T. Sato, and K. Watanabe. 2017. Hairworm infection and seasonal changes in paratenic hosts in a mountain stream in Japan. Journal of Parasitology 103: 32–37. doi: 10.1645/15-887
Zanca, F., C. De Villalobos, A. Schmidt-Rhaesa, M. G. Bolek, et al. 2020. Phylum Nematomorpha. In J. Thorp, C. Damborenea, and D. C. Rogers, eds. Keys to Neotropical and Antarctic Fauna: Freshwater Invertebrates, Volume 5. Academic Press, Cambridge, Massachusetts, United States.
Supplemental Reading
De Villalobos, L. C., A. Rumi, V. Núñez, A. Schmidt- Rhaesa, et al. 2003. Paratenic hosts: Larval survival strategy in Paragordius varius (Leidy, 1851) (Gordiida, Nematomorpha). Acta Parasitologica 48: 98–102.
Warren, M. B., H. R. Dutton, N. V. Whelan, R. P. E. Yanong, et al. 2019. First record of a species of Memrmithidae Braun, 1883 infecting a decapod, Palaemon paludosus (Palaemonidae). Journal of Parasitology 105: 237–247. doi: 10.1645/18-168

