1 Chapter 1: Introduction to Animal Parasitology
Introduction
One of the most fascinating things that a person can experience in the complex realm of biology is the discovery of an animal living inside another animal. If this discovery takes place at an early enough stage in the development of a young person’s view of the world, that is, before the rules and regulations of what of society thinks, and before what is good and what is bad are perfused into a learner’s mind, the first discovery of living-motile trematode worms living inside the lungs of a frog or of tapeworms inhabiting the gut of a rodent can be exhilarating and a positively unforgettable experience. The questions that arise when these kinds of animals are encountered for the first time are innumerable and, if answered carefully and perhaps fully, may lead to more and more questions, and hopefully, more and more answers.
Many students of biology first begin to investigate parasites and parasitism via the initial study of the ecology, behavior, or systematics of a species of a free-living organism. That is, the free-living animal (pick your favorite species) is being studied for any of a myriad of reasons and during the investigations, those doing the work discover that there maybe several species of parasites occurring in or on (or, more likely, both) their study animals. This discovery can occur for other reasons not related to parasitology at first, but then leads to investigation of parasitism.
The parasitic way of life is one of the most common—if not the most common—way of protozoan and animal life that exists. It is likely that more than half of all species of organisms are parasites, and many are of very great economic and medical importance. Some of the most devastating diseases of humans, such as malaria, trypanosomiasis, and filariasis, are caused by parasites, and the economic loss caused by parasites of plants and animals worldwide reaches the equivalent of billions of United States dollars every year.
Definition of a Parasite
The concept of a parasite and its host essentially refers to the biological tension between 2 organisms that live physically adjacent to one another. With the classical definition of a parasite as an organism living on or in another organism (the host) and usually causing some harm to the host, the parasite sounds like it is merely a bad thing with respect to the host; and this definition works for the most part since most parasites probably do harm the host. In some species, the harm can be minuscule and undetectable, without causing discomfort to the host, or the damage can be significant, actually killing the host. For example, pinworm nematodes probably don’t do very much to decrease the ability of their hosts to go about their daily lives or produce a normal number of offspring and live to old age. On the other hand, species of the phylum Acanthocephala, known as the thorny-headed worms, can cause a great deal of harm to their definitive or final hosts by penetrating the mucosal layer of the small intestine with their proboscis and sometimes the proboscis may penetrate the muscularis mucosa through the serosa into the peritoneal cavity, causing peritonitis, and when this occurs, the host usually dies.
Parasitism, beyond the classical definition provided above, can be defined in a very wide sense, that is, as a close association between 2 organisms, in which a parasite depends on a host that provides some benefit to it (usually nutrition or food, depending on the group of parasites), and the parasite does not always damage the host (as noted above, pinworms of rodents are good examples of this). A parasite can be very small relative to the size of the host— and most parasites are much smaller than the host; however, some parasites can reach huge sizes, and those that become numerous or are very large can even drain their host’s blood of essential nutrients.
Parasitology is usually restricted to single celled eukaryotic, or protozoan (also called protistan) and multicellular or metazoan parasites, whereas many groups of organisms that lead a parasitic way of life, such as some fungi and bacteria, are usually instead included in the domain of microbiology, while viruses are studied in virology. However, it really depends on convention. In France, for example, fungi are often studied by parasitologists in addition to the helminths and protozoans or protistans.
Different authors use different definitions for parasitism, depending on their perspective or research interests. Thus, a medical parasitologist will stress that a parasite causes certain diseases and will exclude certain species from the definition which have no apparent ill effect on the host. A zoologist might be more interested in the physiological and morphological adaptations of a parasite to its host or of the host to its parasite. An ecologist may be more interested in the interactions of the parasite on its host and the animal populations with which parasites live, while an evolutionary biologist may be interested in the evolutionary interactions among parasites and their hosts without too much regard for the individual species of animals that are being studied. The definitions presented here are from the general perspective of a parasite systematist, one who is primarily concerned with the understanding of parasitism from the aspect of parasite biodiversity, how they evolved and are evolving, and any and all relationships among them (and their hosts).
Associations Related to Parasitism
Some types of ecological associations resemble parasitism in various aspects and cannot always be unambiguously distinguished from a parasitic relationship, either because little is known about a particular species or because intermediate forms exist. Such ecological associations include: Predation, commensalism, phoresis, mutualism, and symbiosis sensu stricto (meaning, in the strict sense). In the case of predation, the predator usually kills and eats another animal, the prey. In the case of commensalism, an organism associated with a host uses food found in the internal or external environment of the host and there may be no close phylogenetically determined relationship with the host or host group. For example, many species of barnacles and isopods can take up residence on the external surfaces of whales. These can then be termed ectocommensals (ecto = outside of the host). In phoresis, one organism uses another only for transport and/ or protection. Barnacles can again serve as an example: Some species live attached to the skin of whales, by which they are carried around finding new sources of pelagic food (plankton). A mutualistic association is one in which both host organism and the associated species benefit. The Australian mistletoe bird Dicaeum hirundinaceum feeds on the seeds of mistletoes which are plants that derive most of their sustenance from their host plants, and the mistletoe depends on the bird for dispersal of its seeds through space. Symbiosis (sensu stricto) is an extreme form of mutualism, in which the association is compulsory, that is, both partners (symbionts) benefit and cannot live without each other. Very ancient examples of symbiosis are organelles (specialized cell components) of all protozoan (unicellular) and metazoan (multicellular) animals and plants, which are thought to have arisen by the joining of originally free-living organisms. However, the term symbiosis is also occasionally used in a wider sense that can include the phenomena of parasitism, commensalism, phoresis, and mutualism.
That a distinction between the various kinds of associations is sometimes difficult to make is shown by the observation that the same organism may sometimes be a parasite, commensal, mutualist, or predator, depending on the circumstances. Thus, oftentimes, the amoeba Entamoeba histolytica may feed on bacteria in the intestine of humans without causing any damage, or it may live as an often-fatal pathogenic parasite ingesting red blood cells and sometimes penetrating through the gut wall into the abdominal cavity, with fatal consequences. Some parasites may even improve the well-being of their hosts when infection intensities are low, but this is an understudied area.
Kinds of Parasitism
Lice, ticks, fleas, some monogeneans, and many crustaceans such as isopods and barnacles, as alluded to above, are ectoparasites that live on the surface of animals. Nematodes (such as species of Oxyurida or Oxyuroidea), tapeworms (such as fish, beef, and pork tapeworms), flukes (also known as trematodes, such as liver flukes, eye flukes, and blood flukes), and coccidian parasites (such as Plasmodium, which causes the disease malaria in humans) are examples of endoparasites found in the tissues or within the organs of their hosts. Cestodes and trematodes are obligate parasites which cannot survive without a host at least for part of their life cycle, whereas some maggots (larvae of flies that usually feed on decaying organic matter) may be facultative parasites, which infect living animals only occasionally (note that there are plenty of species of flies in which their larval stages are parasitic in vertebrates and cannot live anywhere else). Permanent parasites, such as most parasitic helminths, including trematodes, cestodes, and nematodes, are organisms that are parasitic on or in a host over long time spans, whereas temporary parasites, such as most leeches, are parasitic only intermittently.
An example of a sexually dimorphic parasite is the chigoe flea Tunga penetrans Linnaeus in which only the female is a permanent parasite—usually on the toes of some hapless human or some other mammal—and the male may move around from toe to toe and from host to host. Some species of parasites are selective in their parasitic existence such as species of the phylum Arthropoda that range in diversity from marine gnathiid isopods (phylum Arthropoda: sub-phylum Crustacea: class Isopoda) to terrestrial chigger mites (class Acari: family Trombiculidae). Some species in these 2 groups are parasites only as larvae, thus they are referred to as larval parasites. In this example, the isopod larvae live on marine fish and suck their blood, yet when they molt to the adult stage they live the rest of their lives eating detritus in the benthic zone of the sea floor. The trombiculid mites (family Trombiculidae) exist as adults that eat detritus in the soil and they lay eggs there that hatch into larvae called chiggers that are the torment of humans and other mammals worldwide. Other larval parasites include the cysticercoids of hymenolepidid tapeworms (phylum Platyhelminthes: class Cestoda: family Hymenolepididae) that live in mites or beetles as larvae and mature to adults in their rodent final or definitive hosts. However, many organisms are parasitic only as adults and they are associated with a host for all, or at least part, of their sexually reproductive phase.
Female mosquitoes and some fly larvae like the Congo floor maggot (Auchmeromyia luteola; see Zumpt, 1965) are periodic parasites which visit a host periodically. In this example, the A. luteola maggot comes out of its daytime hiding place in the evening and fills up on the blood of a sleeping human, and then goes back into the floor to wait until the next feeding session. When individuals of the same species parasitize other individuals of the same species, they are referred to as intraspecific parasites. This type of parasitism is not very common but does occur. An example is that of males of some deep sea fish that live permanently attached to females of the same species, absorbing food and deriving physical protection from the female. Hyperparasites (of the primary, secondary, tertiary, etc. degrees) are parasites of other parasites. For example, some protozoans infect helminths (worms) in the intestine or tissues of fishes, and this also occurs in nematodes that have flagellated protozoa (Histomonas meleagridis) in the uterus of females that are actually transmitted to the next galliform bird host such as chickens and turkeys (class Aves: order Galliformes) and are protected in the eggs of the nematode. Kleptoparasites are animals which force others to regurgitate or drop their food and then steal and eat their prize, and this is an example of behavorial parasitism. Frigate birds and some hawks chase other birds in flight. Cowbirds and about 50 species of cuckoos are brood parasites, that is, they lay their eggs in the nests of other birds where they are incubated by and cared for by the parental birds of the nest they have invaded. Microparasites include viruses, bacteria, protozoans, and some small worms (helminths), which reproduce in or on the host, sometimes inducing immune responses in vertebrate hosts. Macroparasites, that is, large-bodied parasites, include most helminths and arthropods; most do not multiply within the host. There are many species of hymenopterans (phylum Arthropoda: class Insecta: order Hymenoptera) that are considered parasitoids. These are animals that lay their eggs in insect or other arthropod hosts and the egg hatches and begins to feed on the host tissues. Here, the host may survive for some time before it is eventually killed by the feeding and growing larval parasitoid. In some cases, several levels of hyperparasitism have been identified in which parasitoids are parasitized, such as by a wasp.
Mechanisms of Infection
Specific mechanisms of infection are truly numerous and are well-studied in many species of parasites (Table 1). Some species of parasites possess conspicuous morphological adaptations that increase the probability that the life cycle will be completed. For example, eggs of some blood flukes of humans (namely, schistosomes causing schistosomiasis also known as bilharzia or bilharziasis) have spines which contribute, together with enzymes produced by the larva within the egg, to eroding the walls of blood vessels where the adults live, thus facilitating escape of eggs produced by the female directly into the bloodstream. The eggs then travel from the bloodstream through the walls of the blood vessels into the feces or urine, depending on the species of Schistosoma (adults of S. haematobium live in blood vessels around the urinary bladder while adults of S. mansoni live in the blood vessels of the intestines).
Adaptations to Parasitism
Each parasite species has adaptations that increase the probability of the parasite to infect, or make it to, a new host and increases the chance of survival in it. For example, Plasmodium species in birds cannot normally survive in primates, and the species of human pinworm (oxyurid nematode) Enterobius vermicularis is known only from humans, although other species of Enterobius occur in primates with 1 species being reported from rodents (Brooks and McLennan, 2002). In other words, each of these species possesses characteristics enabling it to complete its life cycle using these hosts. Such characteristics (in the very few cases analyzed in some detail) determine not only the species of host(s) used, but also the degree of host range, that is, how many host species a parasite can utilize (Brooks et al., 2022).
Like all animal species, parasites must be able to disperse, as populations with a small numerical density and limited geographic distribution may be at risk of extinction when environmental conditions become unfavorable or they may succumb to inbreeding depression via loss of genetic heterozygosity, and (perhaps) run the risk of overinfecting a local and restricted animal-host population. In parasites, dispersal may be mostly, or even entirely, passive; that is, the parasite is spread to new geographic areas and new hosts via the geographic dispersal of the host. Many parasites have elaborate dispersal mechanisms, such as flotation organs of larval flukes (cercariae), polar filaments on the eggs of some cestodes that live in water birds, and some parasites can even modify the behavior of their host to increase the probability that the parasite will make it to the next host.
Aggregation, Hermaphroditism, Parthenogenesis, and Asexual Reproduction
Surveys of the distribution of parasites in animal populations always find that not all potential host individuals are infected to the same degree. Most parasites are usually concentrated in a few individuals of the host population. This is what is meant by distributions being aggregated or over-dispersed. There has been some debate about whether aggregation has a biological function, such as facilitating the finding of mates, or limiting the damage done overall to the host population. Statistically speaking, in the negative binomial distribution, the variance is greater than the mean, so the variance divided by the mean is greater than 1. Since these are counts of numbers of parasites in hosts that were examined, the fact that few hosts have many parasites shows an overdispersed or an aggregation distribution of the parasites in or on a few hosts. The parasites are not dispersed evenly throughout the host population. Whenever the variance/mean is greater than 1, it is said that the distribution is overdispersed or aggregated.
Overdispersion characterizes a phenomenon of aggregation of a majority of parasites in a minority of the host individuals in a certain population. Thus, the majority of hosts have no or few parasites. A very small number of hosts, however, carry a great number of parasites. Crofton (1971) first showed that overdispersion was present for parasite populations. Since then, overdispersion has been defined as axiomatic among parasites of a variety of vertebrate and invertebrate hosts (Knight et al., 1977; Anderson and May, 1985; Crompton et al., 1984). Patterns of overdispersion have also been discovered in populations of managed species of wildlife (Shaw et al., 1998; Wilson et al., 2002).
Additional research shows that the same general pattern occurs across several other species of animals. For example, cestodes of the species Triaenophorus nodulosus (class Cestoda: family Bothriocephalidea) in perch fish (Perca fluviatilis) show less aggregated distributions with only 54% of these worms occurring in 18.5% of hosts with 81.5% of fish remaining uninfected or lightly infected. Data accumulated relative to infections by the nematode Porrocaecum ensicaudatum (phylum Nemata: superfamily Ascaridoidea) in populations of the European starling (Sturnus vulgaris) from 1 study, 89% of the hosts were uninfected or lightly infected, and 69% of the parasites were recorded in just a few (11%) of the hosts. In pond frogs Rana nigromaculata harboring nematodes of the species Spiroxys japonica (phylum Nemata: class Spirurata: family Gnathostomatidae), it was found that 70% of the parasites were recorded in just 4% of the frogs examined while 88% of the frogs were found to be uninfected and 8% had light infections (Shaw et al., 1998).
Overdispersion was also recorded for 4 species of the most common human-infecting geohelminths (Croll and Ghadirian, 1981) and a search of the literature shows that almost invariably, parasites are distributed through animal populations in a non-random way, but what determines this is still poorly understood. For summaries of this topic in helminth parasites, see Churcher et al. (2005) and Lester (2012).
General Reproductive Biology of Parasites
Common among parasites are the various methods of reproducing that are found in the Kingdom Animalia, includ- ing: Hermaphroditism (1 individual has fully functioning male and female organs), parthenogenesis (females are able to produce offspring without mating), and asexual reproduction (an individual reproduces by budding or spores in which there is no recombination of genes on the chromosomes). Thus, in asexual modes of reproduction, the resulting new individuals are clones of the original organism. Among most species of parasites, only a single individual or very few in- dividuals will reach and successfully infect or colonize a new host. In this case, populations of parasites may establish and then increase in numerical density from just a few founder individuals, or even from a single founder individual, that makes it to a new animal that it can then utilize as a host. It is a paradigm of evolutionary theory that sexual reproduction creates new combinations of genes that provide the raw material for evolution via natural selection (Williams, 1966; Williams, 1992). However, in reproduction that requires no mating and thus no sexual recombination of genes via the mixing of chromosomes, the advantage of rapid population growth from a single propagule in a new environment may in the short term outweigh the advantages of sex (Ghiselin, 1969; Williams, 1992; Kearney, 2022).
An example of asexual reproduction in a parasite occurs in species of Plasmodium (the causative agent of malaria in people). This example illustrates the stage that occurs in the vertebrate intermediate host, in the red blood cells after the infective stages first multiply in liver hepatocytes and are released into the bloodstream. In the bloodstream, these parasites develop in the red blood cells (RBCs) and multiply by mitotic division of the nucleus and other cell organ- elles but not the cytoplasm. These then escape the RBCs into the bloodstream to invade more RBCs and undergo more cycles of development and multiplication (depending on the species).
Parasitic platyhelminths, including trematodes, cestodes, and monogeneans, with a few exceptions, are hermaphroditic and individuals can, if necessary (such as when there are no mates nearby), fertilize their own eggs, although they usually cross-fertilize due to several morphological and developmental stages that decrease probability of self-fertilization in these groups. Some species of nematodes, including those in the genera Steinernema and Heterorhabditis (entomopathogenic nematodes, namely, those that infect insects as part of their life history) also have hermaphroditic stages (Cao et al., 2022). Many other species have been shown to exhibit various methods besides sexual reproduction and some of these are reviewed in Triantaphyllou and Hirschmann (1964) and Maggenti (1981).
Parthenogenesis is the growth and development of an animal from an ovum without fertilization and this occurs commonly in species of Strongyloides (phylum Nemata: family Strongylidae) which infect mammals (see Cable, 1971; also see the definition of parthenogenesis in Maggenti, 1981).
Host Range
Some parasites are known to occur in or on a few or, in some cases, only 1 species of free-living animal. Definitions are always problematic, and defining species of parasites with limited host range (formerly, or at times still, referred to as host specificity) depends on vast knowledge that can only be based on extensive collections of animals conducted over broad geographic spaces and includes complete data for the specimens of both parasites and their hosts (note that if an animal is not parasitized, it is not a host, but is only a potential host). In order for these data to be useful, the specimens that are collected and processed and their associated data must be deposited in museums that maintain both specimens and their data in perpetuity. The reason that the host and parasite are both stored in museums after collection is to enable tests of the hypotheses of host-range by actually looking at, and using data for, both the host and parasite. Many times, the host group is misidentified in the field and the species name can only be positively known by comparative methods using museum collections (Brooks et al., 2015; Galbreath et al., 2019).
Most species of parasites show some level of limited host range, although the extent of limits among species is variable. For example, the large human nematode Ascaris lumbricoides (phylum Nemata: order Ascaridida) has a di- rect life cycle and occurs in both humans and pigs (Araújo et al., 2015). The apicomplexan protozoan Toxoplasma gondii (phylum Apicomplexa: family Sarcocystidae) has been shown to occur in a wide range of mammals and birds and shows broad infectivity on those groups of potential hosts (Dubey, 2008).
Attempts to understand patterns of diversity of parasites that have both wide and narrow host ranges have been on-going with concentrated work and summaries presented first by Baer and Mayr (1957). This work has been one of the foundations of systematic and ecological parasitology since the beginning of the scientific study of parasites (Guerrero, 2021; Hoeppli, 1959); however, the collections of individual parasites from vertebrates representing myriad species and their deposition into museums (as well as depositing individual host animals) has not kept pace with the same work on the vertebrates themselves (Galbreath et al., 2019). In a summary of mammal collections in museums in the United States (Dunnum et al., 2018), there were estimated to be about 5,275,000 individual cataloged mammal specimens distributed through 395 active mammal collections. However, there are only a handful of major collections of parasites of mammals in the United States and, of those, only 2 collections have significantly large reciprocal collections of both mammals and the parasites that were found during geographically focused surveys and inventories of the mammals themselves. Thus, without excellent reciprocal collections of parasites and their hosts with their data available in museums, it is difficult to say very much about host range. Until more data are collected, certain questions will remain unanswered.
Species Richness of Parasites and Distribution of Parasites
Arndt (1940) was the first ecologist who counted the number of parasites as a proportion of a total fauna. In Germany, he found 10,000 parasitic species out of a total of 40,000 species, but did not include insects parasitizing plants, as he classified them as herbivores. Price (1977) included such species but excluded temporary parasites (for example, mosquitoes and leeches) in his survey of the British fauna. Price estimated that more than half of all British species are parasitic.
Thirteen large taxa (phyla, subphyla, or classes) consist entirely of parasites, and many other groups include a high proportion of parasitic species. Even among the vertebrates several species are parasitic, such as the sea lamprey Petromyzon marinus.
Virulence of Parasites
Virulence of parasites can be defined as the degree of damage done by the parasite to the host. There are 2 opposing trends which determine the degree of virulence: 1) Usually it is not a selective advantage to severely damage or kill its host, because this would also affect the fitness of the parasite; 2) parasite transmission to another host may be facilitated by such damage: A weak host may be easier prey for a predatory final host than a strong one. Therefore, evolution will lead to an increase or a decrease in the virulent nature of various parasites, depending on the circumstances.
Life Cycles
Many parasites have a direct life cycle (lice, fleas, monogenean flukes, and many nematodes) and they use a single host which harbors larval/juvenile stages as well as adults. Other parasites have complex life cycles and use a final (= definitive) host which harbors the mature stage, as well as 1 or several intermediate hosts which harbor the larvae, that is, they have indirect life cycles (for example all digenean flukes, all of which are from class Trematoda). An example of a trematode with 2 intermediate hosts is the lancoleate trematode Dicrocoelium dendriticum. In certain parasite species, alternative life cycles are possible. For example, in the aspidogastrean fluke Aspidogaster conchicola, both a direct and an indirect life cycle are possible: Adult worms in the mollusc produce eggs which are inhaled by other molluscs, but fish can also become infected by eating infected molluscs. In other aspidogastreans, and in the amphilinid tapeworm Austramphiina elongata, among many others, the life cycle is always indirect, involving both an intermediate and final host. In the amphilinid tapeworm, turtles serve as final hosts, eggs escape from the host in an unknown way, larvae hatch in freshwater and penetrate into a crayfish intermediate host, which is then eaten by a turtle.
Many species of parasites possess varied and diverse behavioral adaptations that facilitate completion of their life cycle and entrance into the next host in the cycle. Adult Dicrocoelium dendriticum (phylum Platyhelminthes: class Trematoda: subclass Digenea) infect the liver mainly of sheep, but other ungulates are also parasitized by these trematodes. These trematodes produce eggs which pass out of the host with the feces and are eaten by land snails, in which various larval stages are produced. The last stage is the tailed larva, or cercaria, many of which cluster in slime balls which are left behind in the mucus trail of the snail as it speeds to its objective, whatever that may be. If the trematode larvae are lucky, these slime balls are then eaten by ants. If not eaten, they dry up and die. After being ingested, the cercariae move from the intestinal tract to various parts of the ant. The first cercariae getting into an ant penetrate into the ant’s subesophageal ganglion, inducing the ant to climb up a grass stem and, when the temperature drops, the cercaria induces cramp-like behavior in the ant, which consequently clings to the grass stem with its mandibles. This behavior increases the likelihood of the infected ant being eaten by a passing sheep or another ungulate.
Host-Parasite Interactions, Example: Cleaning Symbiosis
A considerable range of behavioral patterns leading to (or thought to lead to) the removal of parasites has been observed among animals. They include preening and bathing of birds in dust and water, and passive and active anting, where ants are allowed to passively crawl over the body, or where ants are actively squeezed over the plumage. Also, dogs rubbing their skin against rough surfaces, jumping of fish and whales out of water, and so on, may have a cleaning function. Best known is cleaning symbiosis, in which one animal (the cleaner) cleans another (the host) from parasites and diseased (necrotic) tissues. For example, cleaning behavior has been observed in birds which remove ectoparasites from cattle, hippopotamuses, large marine fish floating on the ocean surface, several species of shrimp, and some freshwater fish. Hosts are freshwater and marine fishes, whales and dolphins, and invertebrates, among others. Many cleaner fish possess special morphological adaptations which enable them to pick parasites off of the host skin or even gills (the mouth is located terminally to facilitate picking up of parasites, the anterior teeth are fused to form cutting plates, and color patterns are conspicuous, useful in signaling to hosts: “I am a cleaner!”). The cleaner fish Labroides dimidiatus even performs a cleaning dance to attract host fish. Invitation postures of hosts signal, in turn, to the cleaner that they are ready to be cleaned.
Capacity
What is meant by capacity? As noted earlier, every species, including all parasites, have specific environmental re- sources they need in order to survive and reproduce. In the case of parasites, those resources are specific attributes of their hosts. For a given parasite species, if only 1 host species has the required resources, the parasite can survive only in association with that species, and its survival is tied to the survival of that species. But the vast majority of inherited attributes of all species are evolutionarily conservative, meaning they occur in more than 1 species of host.
All parasites live in association with a restricted number of hosts, and some not so restricted, as seen in Toxoplasma, and sometimes only 1 host species is infected. Sometimes parasites are restricted to a potential or actual species of host by limited capacity but mostly parasites are restricted by limited opportunity. And so, when the conditions change—say, as a result of climate change or intrusion of humans and their domestic animals into previously uncut forest—new opportunities are created and the parasites move into hosts they had the capacity to infect but never before had the opportunity to (this could be the result of trophic changes locally or of geographic dispersal into new areas).
Ecological Fitting
Ecological fitting (sensu Janzen, 1985) refers to cases when a parasite has the opportunity to encounter a new potential host that has the required resources for survival, the parasite will then be expected to add that species to its repertoire. This, by the way, eliminates the need for the right mutation to show up at the right time to allow or enable the parasite to jump into a new species of animal to make it a new host.
Fundamental Host Fitness Space
For any given parasite, the range of all hosts that have the required resources is called the fundamental host fit- ness space (in accordance with Hutchinson’s notion of fundamental niche space; Hutchinson, 1959), which Agosta called fundamental fitness space in order to relate it directly to evolution (Agosta, 2006; Agosta et al., 2010). The actual hosts inhabited by the parasite at any given time represent the realized host fitness space (in accordance with Hutchinson’s realized niche space and Agosta’s use of the term fitness rather than niche). One of the keys to the evolutionary success of parasites is that the fewer species of animals used as actual hosts (that is, the smaller the realized fitness space), the more potential opportunities to inhabit new species of hosts exist. In other words, given the opportunity to come into contact with a suitable but previously unexposed (potential) host species, a parasite would add the new host to its host range and survive even if the original species of host went extinct. The fewer hosts actually used, the smaller the proportion of actual host fitness space compared to fundamental host fitness space and consequently the sloppier (meaning, more filled with potential opportunities) the host fitness space. At the same time, the more restricted the realized host fitness space, the more specialized the parasite is within that fitness space. Alternatively, the more species of potential hosts used, the larger the proportion of actual host fitness space compared to fundamental hosts space, the less sloppy the fitness space, the fewer new potential hosts, and the more generalized the parasite is in fitness space. This insight, developed by Agosta (2006) and elaborated by Agosta et al. (2010) and Brooks and Agosta (2020), obviates the need to define or even discuss host specificity since it is basically impossible to look at host specificity in an evolutionary sense. Conversely, the idea of fitness space has a Darwinian evolutionary origin that can be tested in an evolutionary context.
Economic and Hygienic Importance of Parasites
Some of the most important tropical diseases of humans are caused by parasites, such as schistosomiasis (bilharziasis) (caused by the blood fluke Schistosoma), filariasis (caused by several different species of filarioid nematodes), amebic dysentery (the protozoan Entamoeba histolytica is the causative agent of this one), and, in particular, malaria (at least 5 species of the protozoan Plasmodium). Annually, more than 247 million people are infected with various species of Plasmodium, the causative agent of malaria, and around 619,000 people die from it every year worldwide, particularly children in sub-Saharan Africa. The webpages of the World Health Organization (WHO), Division of Tropical Diseases and of the United States Centers for Disease Control and Prevention (CDC) contain information about the current status of the important parasitic diseases, which is continually updated. Information on prevalences of infection with various parasites and their geographical distribution are available at the CDC web site (https://cdc.gov) and at the WHO site (https://platform.who.int/mortality/themes/theme-details/topics/topic-details/ MDB/infectious-and-parasitic-diseases). Global warming will lead to a spread of parasitic infections into some countries and increase prevalences of parasites in others that already have high parasite loads in their populations, especially in tropical and subtropical regions that will continue to warm over the next few hundred years (Brooks et al., 2019).
Parting Thought
The rest of this book provides an in-depth overview of many species of parasites, how they are related to one an- other, their adaptations, effects on hosts, and their importance as fellow inhabitants on Earth. This introduction is fittingly ended with a quote from Harold W. Manter (Figure 2), one of the leaders in parasitology from the late 1920s through 1970 and the namesake of the Harold W. Manter Laboratory of Parasitology, one of the world’s leading laboratories of systematic parasitology. Manter was an early proponent of the mutability of continents and plate tectonics and worked to provide evidence of continental movement with data from parasites and their hosts. From this work, he proposed the idea of parascript (Brooks and McLennan, 1993). Extracted from the book Host-Parasite Relationships (McCauley, 1966), Manter stated: Thus, parasites reflect both current environmental conditions and also the influences of ancient times—both ecology and phylogeny … Parasites of fishes, particularly such an abundant and diverse group as the Trematoda, furnish information about present-day habits and ecology of their individual hosts. These same parasites also hold promise of telling us something about host and geographical connections of long ago. They are simultaneously the product of an immediate environment and of a long ancestry reflecting associations of millions of years. The messages they carry are thus always bilingual and usually garbled. Today, we know only a few selected pieces of the code. As our knowledge grows, studies based on adequate collections, correctly classified and correlated with knowledge of the hosts and life cycles involved should lead to a deciphering of the messages now so obscure. Eventually there may be enough pieces to form a meaningful language which could be called PARASCRIPT: The language of parasites which tells of themselves and their hosts both of today and yesteryear.
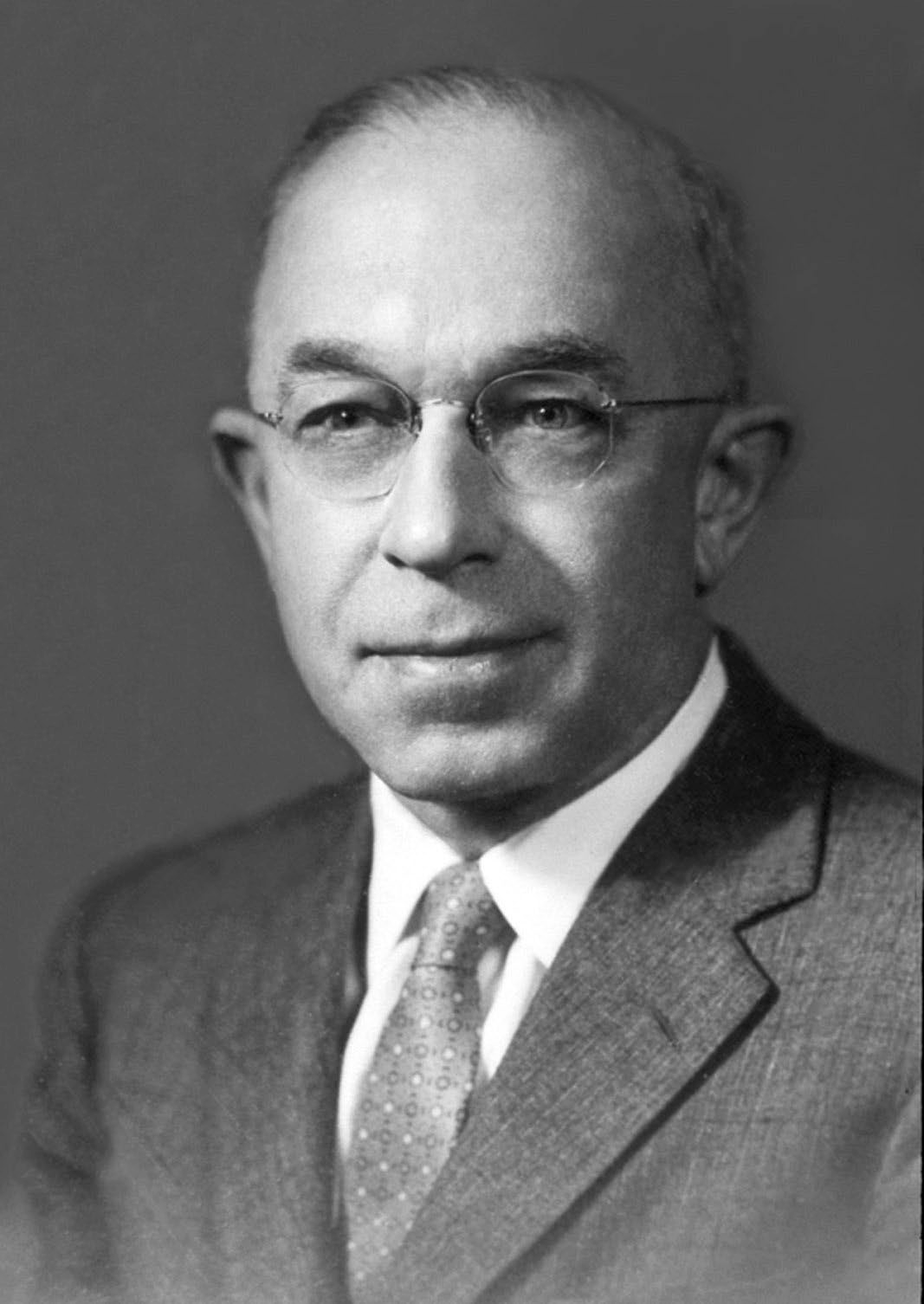
Figure 2. Harold Winfred Manter (1898–1971), circa 1960. Manter was a professor in the Department of Zoology, University of Nebraska (Lincoln campus; Lincoln, Nebraska, United States) from 1925 to 1971. He worked on systematics and biogeography of parasites of fishes, although during his tenure at Nebraska, he trained dozens of students in other areas of parasitology. The Harold W. Manter Laboratory of Parasitology (HWML) was named after him, having been established after his death in 1971 by Curator of the Parasitology Division of the University of Nebraska State Museum, Mary Lou Hanson Pritchard. Source: HWML. License: CC BY.
Acknowledgment
Parts of this work first appeared at https://krohde.word-press.com/2010/10/16/parasitism-an-introduction-to-xk-923bc3gp4-51/, Ecology and Evolution, Parasitologie/Parasitology, prepared by Klaus Rohde in German and English versions, and posted online on October 16, 2010. With Dr. Rohde’s direct input, it was later adapted and expanded by Scott L. Gardner and Daniel R. Brooks for inclusion in the Concepts in Animal Parasitology open access textbook.
Original Text Book Chapter 1.
Authors
Scott L. Gardner
Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States; and School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, Nebraska, United States slg@unl.edu
Daniel R. Brooks
Harold W. Manter Laboratory of Parasitology, University of Nebraska State Museum, Lincoln, Nebraska, United States dnlbrooks@gmail.com
Klaus Rohde
Department of Zoology, School of Environmental and Rural Science, University of New England, Armidale, New South Wales, Australia
krohde@une.edu.au
Revisions: Some sections were removed or reduced for length.
Literature Cited
Agosta, S. J. 2006. On ecological fitting, plant-insect associations, herbivore host shifts, and host plant selection. Oikos 114: 556–565. doi: 10.1111/j.2006.0030-1299.15025.x
Agosta, S. J. 2022. The Stockholm Paradigm explains the dynamics of Darwin’s entangled bank, including emerging infectious disease. Manter: Journal of Parasite Biodiversity 30. doi: 10.32873/unl.dc.manter27
Agosta, S. J., and J. A. Klemens. 2008. Ecological fitting by phenotypically flexible genotypes: Implications for species associations, community assembly and evolution. Ecology Letters 11: 1,123–1,134. doi: 10.1111/j.1461-0248.2008.01237.x
Agosta, S. J., N. Janz, and D. R. Brooks. 2010. How specialists can be generalists: Resolving the parasite paradox and implications for emerging infectious disease. Zoologia (Curitiba) 27: 151–162. https://www.scielo.br/j/zool/a/rZ43L gGRhsbZLjdWsK85X7r/?lang=en
Anderson, S. 1997. Mammals of Bolivia: Taxonomy and distribution. Bulletin of the American Museum of Natural History 231, 252 p. https://digitallibrary.amnh.org/ handle/2246/1620
Anderson, R. M., and R. M. May. 1985. Helminth infections of humans: Mathematical models, population dynamics, and control. Advances in Parasitology. 24: 1–101. doi: 10.1016/ S0065-308X(08)60561-8
Araújo, S. B., M. P. Braga, D. R. Brooks, S. J. Agosta, et al. 2015. Understanding host-switching by ecological fitting. PLoS One 10: e0139225. doi: 10.1371/journal.pone.0139225 Arndt, W. 1940. Der prozentuelle Anteil der Parasiten auf und in Tieren im Rahmen des aus Deutschland bisher bekannten Tierartenbestandes. Zeitschrift für Parasitenkunde 11: 684– 689. doi: 10.1007/BF02120750
Baer, J. G. 1951. Ecology of Animal Parasites. University of Illinois Press, Urbana, Illinois, United States, 224 p.
Baer, J. G., and E. Mayr. 1957. In Premier symposium sur la spécificité parasitaire des parasites de vertébrés. Attinger, Neuchatel, Switzerland, 824 p.
Brooks, D. R. 1985. Historical ecology: A new approach to studying the evolution of ecological associations. Annals of the Missouri Botanical Garden 72: 660–680. doi: 10.2307/2399219
Brooks, D. R., and S. J. Agosta. 2020. The Major Metaphors of Evolution: Darwinism Then and Now. Springer Nature, Cham, Switzerland, 273 p.
Brooks, D. R., and D. A. McLennan. 2002. The Nature of Diversity: An Evolutionary Voyage of Discovery. University of Chicago Press, Chicago, Illinois, United States, 668 p.
Brooks, D. R., and D. A. McLennan. 1993. Parascript: Parasites and the Language of Evolution. Smithsonian Institution Press, Washington, DC, United States, 429 p.
Brooks, D. R., W. A. Boeger, and E. P. Hoberg. 2022. The Stockholm Paradigm: Lessons for the emerging infectious disease crisis. Manter: Journal of Parasite Biodiversity 23, 10 p. doi: 10.32873/unl.dc.manter22
Brooks, D. R., E. P. Hoberg, and W. A. Boeger. 2015. In the eye of the cyclops: The classic case of cospeciation and why paradigms are important. Comparative Parasitology 82: 1–8. doi: 10.1654/4724C.1
Brooks, D. R., E. P. Hoberg, and W. A. Boeger. 2019. The Stockholm Paradigm: Climate Change and Emerging Disease. University of Chicago Press, Chicago, Illinois, United States, 409 p.
Brooks, D. R., D. A. McLennan, V. León-Règagnon, and E. P. Hoberg. 2006. Phylogeny, ecological fitting and lung flukes: Helping solve the problem of emerging infectious diseases. Revista Mexicana de Biodiversidad 77: 225–233. https:// www.redalyc.org/pdf/425/42577209.pdf
Cable, R. M. 1971. Parthenogenesis in parasitic helminths. American Zoologist 11: 267–272. doi: 10.1093/ icb/11.2.267
Cao, M., H. T. Schwartz, C. H. Tan, and P. W. Sternberg. 2022. The entomopathogenic nematode Steinernema hermaphroditum is a self-fertilizing hermaphrodite and a genetically tractable system for the study of parasitic and mutualistic symbiosis. Genetics 220: iyab170. doi: 10.1093/ genetics/iyab170
Churcher, T. S., N. M. Ferguson, and M.-G. Basáñez. 2005. Density dependence and overdispersion in the transmission of helminth parasites. Parasitology 131: 121–132. doi: 10.1017/s0031182005007341
Crofton, H. D. 1971. A quantitative approach to parasitism. Parasitology 62: 179–193. doi: 10.1017/S0031182000071420
Croll, N. A., and E. Ghadirian. 1981. Wormy persons: Contributions to the nature and patterns of overdispersion with Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus and Trichuris trichiura. Tropical and Geographical Medicine 33: 241–248.
Crompton, D. W., A. E. Keymer, and S. E. Arnold. 1984. Investigating over-dispersion: Moniliformis (Acanthocephala) and rats. Parasitology 88: 317–331.
Dubey, J. P. 2008. The history of Toxoplasma gondii: The first 100 years. Journal of Eukaryotic Microbiology 55: 467–475. doi: 10.1111/j.1550-7408.2008.00345.x
Dunnum, J. L., B. S. McLean, and R. C. Dowler. 2018. Mammal collections of the Western Hemisphere: A survey and directory of collections. Journal of Mammalogy 99: 1,307– 1,322. doi: 10.1093/jmammal/gyy151
Erwin, T. L. 1985. The taxon pulse: A general pattern of lineage radiation and extinction among carabid beetles. In G. E. Bull, ed. Taxonomy, Phylogeny, and Zoogeography of Beetles and Ants. Junk, Dordrecht, Netherlands, p. 437–472.
Galbreath, K. E., E. P. Hoberg, J. A. Cook, B. Armién, et al. 2019. Building an integrated infrastructure for exploring biodiversity: Field collections and archives of mammals and parasites. Journal of Mammalogy 100: 382–393. Includes supplemental material, Field methods for collection and preservation of mammalian parasites, 36 p. doi: 10.1093/ jmammal/gyz048
Gardner, S. L. 1996. Essential techniques for collection of parasites during surveys of mammals. In D. E. Wilson, R. Cole, J. D. Nichols, R. Rudran, et al., eds. Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Smithsonian Institution Press, Washington, DC, United States, p. 291–298.
Gardner, S. L., and J. Whitaker. 2009. Endoparasites of bats. In S. Bernard, ed. Bats in Captivity, Volume 1. Krieger Publishing, Malabar, Florida.
Gardner, S. L., and F. A. Jiménez-Ruiz. 2009. Methods of endoparasite analysis. In T. Kunz and S. Parsons, eds. Ecological and Behavioral Methods for the Study of Bats. Johns Hopkins University Press, Baltimore, Maryland, United States, p. 795–805.
Gardner, S. L., R. N. Fisher, and S. J. Barry. 2012. Field parasitology techniques for use during reptile surveys. In R. McDiarmid, M. Foster, C. Guyer, J. W. Gibbons, eds. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. Smithsonian Publications, University of California Press, Oakland, California, United States, p.114–121.
Ghiselin, M. T. 1969. The evolution of hermaphroditism among animals. Quarterly Review of Biology 44: 189–208. doi: 10.1086/406066
Guerrero, R. 2021. Natterer in Neotropical Nematoda: Species described by Rudolphi, Diesing, and Molin. Manter: Journal of Parasite Biodiversity 18, 55 p. doi: 10.32873/unl. dc.manter17
Hoeppli, R. 1959. Parasites and Parasitic Infections in Early Medicine and Science. University of Malaya Press, Singapore, Singapore, 549 p.
Hutchinson, G. E. 1959. Homage to Santa Rosalía, or why are there so many kinds of animals? American Naturalist 93: 145–159. doi: 10.1086/282070
Janz, N., and S. Nylin. 1998. Butterflies and plants: A phylogenetic study. Evolution 52: 486–502. doi: 10.1111/ j.1558-5646.1998.tb01648.x
Janzen, D. H. 1985. On ecological fitting. Oikos 45: 308–310. doi: 10.2307/3565565
Janzen, D. H. 1980. When is it coevolution? Evolution 34: 611– 612. doi: 10.1111/j.1558-5646.1980.tb04849.x
Kearney, M. R., M. E. Jasper, V. L. White, I. J. Aitkenhead, et al. 2022. Parthenogenesis without costs in a grasshopper with hybrid origins. Science 376: 1,110–1,114. doi: 10.1126/ science.abm1072
Klassen, G. J. 1992. Coevolution: A history of the macroevolutionary approach to studying host-parasite associations. Journal of Parasitology 1: 573–587. doi: 10.2307/3283532
Knight, S. A., J. J. Janovy, Jr., and W. L. Current. 1977. Myxosoma funduli Kudo 1918 (Protozoa: Myxosporida) in Fundulus kansae: Summer epizootiology. Journal of Parasitology 63: 897–902.
Lester, R. J. G. 2012. Overdispersion in marine fish parasites. Journal of Parasitology 98: 718–721. doi: 10.1645/ GE-3017.1
Maggenti, A. R. 1981. General Nematology. Springer-Verlag, New York, New York, United States, 381 p.
Malicka, M., S. J. Agosta, and J. A. Harvey. 2015. Multi-level ecological fitting: Indirect life cycles are not a barrier to host switching and invasion. Global Change Biology 21: 3,210– 3,218. doi: 10.1111/gcb.12928
Manter, H. W. 1966. Parasites of fishes as biological indicators of recent and ancient conditions. In Host Parasite Relationships, Proceedings of the Twenty-Sixth Annual Biology Colloquium, April 23–24, 1965. Oregon State University Press, Corvallis, Oregon, United States.
McCauley, J. E., ed. 1966. Host-Parasite Relationships: Proceedings of the Twenty-Sixth Annual Biology Colloquium, April 23–24, 1965. Oregon State University Press, Corvallis, Oregon, United States, 148 p.
Price, P. W. 1977. General concepts on the evolutionary biology of parasites. Evolution 31: 405–420. doi: 10.1111/j.1558- 5646.1977.tb01021.x
Rausch, R. L. 1993. The biology of Echinococcus granulosus. In Compendium on Cystic Echinococcosis with Special Reference to the Xinjiang Uygur Autonomous Region, the People’s Republic of China, p. 27–56. Brigham Young University Press, Provo, Utah, United States.
Rivas, L. R. 1964. A reinterpretation of the concepts sympatric and allopatric with proposal of the additional terms syntopic and allotopic. Systematic Zoology 13: 42–43. doi: 10.2307/ sysbio/13.1-4.42
Sattmann, H. 2002. Anfänge der systematischen Helminthologie in Österreich. Denisia 6: 271–290. https://www.zobodat.at/ pdf/DENISIA_0006_0271-0290.pdf
Shaw, D. J., B. T. Grenfell, and A. P. Dobson. 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117: 597–610. doi: 10.1017/ s0031182098003448
Thenius, E. 1972. Grundzüge der Verbreitungsgeschichte der Säugetiere. Fischer Verlag, Jena, East Germany.
Thompson, J. N. 2005. The geographic mosaic of coevolution. University of Chicago Press, Chicago, Illinois, United States, 443 p.
Triantaphyllou, A. C., and H. Hirschmann. 1964. Reproduction in plant and soil nematodes. Annual Review of Phytopathology 2: 57–80. doi: 10.1146/annurev.py.02.090164.000421
Williams, G. C. 1966. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought. Princeton University Press, Princeton, New Jersey, United States, 307 p.
Williams, G. C. 1992. Natural selection: Domains, Levels, and Challenges. Oxford University Press, New York, New York, United States, 224 p.
Wilson, K., O. N. Bjørnstad, A. P. Dobson, S. Merler, et al. 2002.Heterogeneities in macroparasite infections: Patterns and processes. In P. J. Hudson, A., Rizzoli, B. T. Grenfell, H. Heesterbeek, et al., eds. The Ecology of Wildlife Diseases. Oxford University Press, Oxford, United Kingdom, p. 6–44.
Zumpt, F. 1965. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists. Butterworths, London, United Kingdom, 267 p.
Supplemental Reading
Erwin, T. L. 1981. Taxon pulses, vicariance, and dispersal: An evolutionary synthesis illustrated by carabid beetles. In G. Nelson and D. E. Rosen, eds. Vicariance Biogeography: A Critique. Columbia University Press, New York, New York, United States, p. 159–196.
Erwin, T. L. 1979. Thoughts on the evolutionary history of ground beetles: hypotheses generated from comparative faunal analyses of lowland forest sites in temperate and tropical regions. In T. L. Erwin, G. E. Ball, D. R. Whitehead, and A. L. Halpern, eds. Carabid Beetles. Springer, Cham, Switzerland, p. 539–592.

